
Improvement in mortality with hyperbaric oxygen therapy in cervical necrotising fasciitis: a systematic review of the literature
Introduction
Necrotizing fasciitis is a soft tissue infection characterised by progressive tissue destruction resulting in significant morbidity and mortality (1,2). Involvement of the head and neck region, although rare, has a particularly high mortality rate due to close relationships to vital structures, rapid spread throughout fascial planes, and communication with the mediastinum (3,4). Cervical necrotising fasciitis (CNF) refers specifically to infection located between the mandible and clavicle, and anterior to the trapezius (5). Mortality of CNF is as high as 25–35% and has decreased over recent years due to early diagnosis and targeted treatment regimens (1,2). Infection of the soft tissue and superficial fascia is most commonly caused by streptococcus species, however wound swabs can often show the presence of polymicrobial organisms with propensity for aerobic, anaerobic or mixed growth (2). The rapidly progressive soft tissue necrosis is thought to occur secondary to both direct tissue infection, as well as obliterative vasculitis resulting in soft tissue ischaemic necrosis (2,6). Disease progression occurs due to host immunosuppression, virulence factors of bacteria, as well as delayed treatment (7).
Treatment of CNF consists of extensive surgical debridement and empiric broad spectrum antibiotics with the goal of addressing all infected and necrotic tissue. Hyperbaric oxygen therapy (HBOT) is currently recommended as a complementary treatment for CNF and achieves its effects through increasing the partial pressure of oxygen within the infected tissues (2,8). It is thought to promote wound healing and attenuate further tissue necrosis through the following mechanisms: (I) increasing tissue oxygen perfusion to allow for continued cellular metabolism and fibroblast proliferation within ischaemic tissues; (II) direct bactericidal effects on anaerobic bacteria; (III) increased production of oxygen free radicals resulting in amplification of the host immune response (2,4,9,10). HBOT is administered via a pressure chamber or mask with 100% oxygen at pressures of 2–3 atmospheres, commonly every 24 hours (6,8-11).
There is currently discordance in the literature regarding the value of HBOT in necrotising fasciitis, particularly in the head and neck region (9,12). There are a limited number of case series in the literature with significant demographic and therapeutic heterogeneity, with a lack of randomised control trials. Using a database of all CNF reported in the literature, we aim to systematically review cases of CNF treated with HBOT to describe the demographic, disease, and treatment data, and determine the effects on patient morbidity and mortality. To our knowledge, this is the largest database of cases of CNF treated with HBOT in the literature. We present this article in accordance with the PRISMA reporting checklist (available at https://www.theajo.com/article/view/10.21037/ajo-23-5/rc).
Methods
Search strategy and study preparation
We completed a literature search of PubMed, Medline (1946 to 2022) and Embase (1974 to 2022). Four main search domains were combined with the Boolean operators “AND” and “OR”. The key words within the first two searches were “cervical” OR “neck”. The second two searches were “necrotizing fasciitis” or “necrotising fasciitis”. Both searches were combined with Boolean operator “AND”. The final search was completed on 20 July 2022, limited to articles from 1974 until July 2022. The bibliographies of the relevant studies were reviewed to identify additional citations. All cases fulfilling the search criteria were reviewed by two reviewers for reference to HBOT usage, and cases with relevant demographic, treatment and outcome data were compiled into a database. Duplicate reports were not included. The study was not registered prior to completion. A review protocol was not used throughout the study. No financial or non-financial support was provided for the study.
Study inclusion/exclusion criteria
Cases with a diagnosis of necrotizing fasciitis involving an anatomical region between the mandible and clavicles were included in the database. This was either the primary site of infection, or as a secondary site of infectious spread from other subsites of the head and neck region or the thoracic region. Only cases that used HBOT as a treatment of CNF were included in the study. Cases that did not use HBOT or did not have a diagnosis of CNF were not included. These were either in case studies or case series. Paediatric cases (<18 years of age) and articles that did not have an English translation available were excluded from final analysis. Eligible articles were screened by title and abstract. Studies deemed relevant were examined by full text, and data extraction was performed. The search, article review and data extraction were completed by two authors (Figure 1). Risk of bias was assessed using the Joanna Briggs Institute checklist standardised tool developed for case reports and series (13) (Appendix 1). Each article was reviewed by two independent authors and the bias assessment tool was completed. Cases of disparity were discussed, and a consensus decision was made if required. Final decision on case inclusion was completed by the primary author (Table 1).
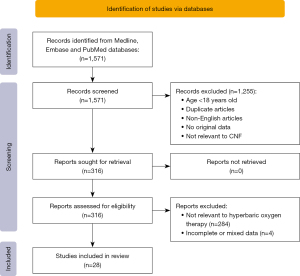
Table 1
| Study | Year | Study type | Question 1 | Question 2 | Question 3 | Question 4 | Question 5 | Question 6 | Question 7 | Question 8 | Question 9 | Question 10 | JBI score | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Krespi (14) | 1981 | Case report | Yes | Yes | Yes | No | Yes | Yes | No | Yes | – | – | 6 | Long-term follow-up unclear |
| Rapoport (15) | 1991 | Case report | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | – | – | 8 | – |
| Gaukroger (16) | 1992 | Case report | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | – | – | 8 | – |
| Jackson (17) | 1995 | Case report | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | – | – | 8 | No clear description of HBOT dose |
| De Backer (18) | 1996 | Case report | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | – | – | 8 | – |
| Ray (19) | 1997 | Case report | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | – | – | 8 | – |
| Burstin (20) | 1999 | Case report | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | – | – | 8 | – |
| Dale (21) | 1999 | Case report | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | – | – | 8 | – |
| Francque (22) | 2001 | Case report | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | – | – | 8 | – |
| Ulubil (23) | 2009 | Case report | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | – | – | 7 | Limited detail of past history and demographics |
| Treasure (24) | 2010 | Case report | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | – | – | 8 | – |
| Chunduri (25) | 2013 | Case report | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | – | – | 7 | Limited follow-up information |
| Gunaratne (5) | 2018 | Case report | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | – | – | 7 | Long-term follow-up unclear |
| Inan (26) | 2017 | Case report | Yes | Yes | Yes | Yes | Yes | Unclear | Unclear | Yes | – | – | 6 | Limited follow-up information |
| Silva (27) | 2020 | Case report | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | – | – | 8 | – |
| Djupesland (28) | 2000 | Case report | Yes | Yes | No | No | Yes | No | Unclear | Yes | – | – | 4 | Unclear diagnostic criteria, not standardised diagnosis, clinical information limited |
| Bayetto (29) | 2017 | Case report | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | – | – | 8 | – |
| Cortese (30) | 2017 | Case report | Yes | No | Yes | Yes | Yes | No | Unclear | Yes | – | – | 6 | Limited presenting time course, limited follow-up information |
| Maisel (31) | 1994 | Case series | Yes | No | No | Unclear | Unclear | Yes | Yes | Yes | Yes | NA | 5 | Nil statistical analysis, diagnostic criteria unclear, unclear if all patients included |
| Langford (32) | 1995 | Case series | Yes | No | No | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes | 6 | Unclear if sequential, diagnostic criteria not the same for all patients |
| Mathieu (33) | 1995 | Case series | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | 9 | – |
| Whitesides (34) | 2000 | Case series | Yes | No | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | NA | 7 | Unclear diagnostic criteria, not standardised diagnosis |
| Krenk (2) | 2007 | Case series | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 | Unclear diagnostic criteria, not standardised diagnosis |
| Flanagan (35) | 2009 | Case series | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 | Unclear diagnostic criteria, not standardised diagnosis |
| Wolf (4) | 2010 | Case series | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 | Unclear diagnostic criteria, not standardised diagnosis |
| Faunø Thrane (9) | 2017 | Case series | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 10 | – |
| Sideris (36) | 2020 | Case series | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes | Yes | NA | 7 | Unclear if cases were sequential, limited statistical analysis |
| Mcgowan (37) | 2021 | Case series | Yes | No | No | Yes | Yes | Yes | Yes | No | Yes | Yes | 7 | Unclear diagnostic criteria, not standardised diagnosis |
JBI, Joanna Briggs Institute; HBOT, hyperbaric oxygen therapy; NA, not available.
Study outcomes
After careful assessment of the included articles, demographic and outcome data was collected of each included patient, and this was collated in our database. This included demographic data, background medical history, and aetiologic source, as well as empiric treatments provided (surgical debridements, antibiotics and HBOT), microbiologic findings, and outcome data (complications, length of stay and mortality). This was collated in our spreadsheet and prepared for statistical analysis. If patients were grouped within the article, then this was represented in the database. If any subset of data was not available, then it was marked as such, and these cases were excluded in statistical analysis.
Statistical analysis
Microsoft Excel 16.64 (Microsoft Corporation, Redmond, WA, USA) was used for data collection and STATA statistical analysis software (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX, USA) was used for formal data analysis. Demographic and outcome data were compiled via standard summary statistical methods. Where individual patient data was available (ungrouped data), cases were added to the spreadsheet as individual cases and subgroup analysis was performed. Where patient data was already combined (grouped data), demographic, treatment and outcome data were presented as means and medians. The pooled proportion of mortality 95% confidence interval was calculated using the Wilson score method (38).
Results
A total of 161 patients who underwent HBOT for CNF were identified in 28 published articles. This included 92 males and 69 females (57% male) with a mean age of 50 years (Table 2). Of the listed comorbidities, diabetes was the most common, present in 20% of cases where documented, and 21% of cases had a documented history of chronic alcohol abuse.
Table 2
| Author | Year of publications | Population size | Age (years)* | Gender (male) | Number of bacteria grown (mean) | Length of stay (days, mean) | Survival (number) | Mortality (%) |
|---|---|---|---|---|---|---|---|---|
| Krespi | 1981 | 1 | 31 | 1 | NA | NA | 1 | 0 |
| Rapoport | 1991 | 1 | 50 | 0 | 4 | NA | 1 | 0 |
| Gaukroger | 1992 | 1 | 76 | 0 | 2 | 50 | 1 | 0 |
| Maisel | 1994 | 4 | 41 | 3 | 2.5 | 29.8 | 4 | 0 |
| Langford | 1995 | 6 | 52 | 4 | 2.5 | 31.6 | 6 | 0 |
| Jackson | 1995 | 1 | 63 | 1 | 3 | 37 | 1 | 0 |
| Mathieu | 1995 | 45 | 42 | 31 | 2.7 | NA | 35 | 22.2 |
| De Backer | 1996 | 1 | 20 | 1 | 4 | 23 | 1 | 0 |
| Ray | 1997 | 1 | 74 | 0 | 1 | NA | 1 | 0 |
| Burstin | 1999 | 1 | 47 | 0 | 1 | 22 | 1 | 0 |
| Dale | 1999 | 1 | 63 | 0 | 3 | 129 | 1 | 0 |
| Djupesland | 2000 | 2 | 37.5 | 2 | 1.5 | 11 | 1 | 50 |
| Whitesides | 2000 | 12 | 50.5 | 7 | 3.3 | 29.9 | 12 | 0 |
| Francque | 2001 | 1 | 44 | 1 | 2 | NA | 0 | 100 |
| Krenk | 2007 | 11 | 59.5 | 3 | 1.1 | 30.8 | 11 | 0 |
| Flanagan | 2009 | 9 | 42 | 4 | 1.3 | 11 | 9 | 0 |
| Ulubil | 2009 | 1 | 27 | 1 | 0 | 17 | 1 | 0 |
| Treasure | 2010 | 1 | 54 | 1 | 5 | 34 | 1 | 0 |
| Wolf | 2010 | 13 | . | 8 | 1 | NA | 13 | 0 |
| Chunduri | 2013 | 1 | 43 | 1 | 2 | NA | 1 | 0 |
| Gunaratne | 2018 | 1 | 43 | 1 | 2 | 56 | 1 | 0 |
| Cortese | 2017 | 2 | 73.5 | 1 | 1 | NA | 2 | 0 |
| Bayetto | 2017 | 3 | 78 | 2 | NA | NA | NA | NA |
| Inan | 2017 | 1 | 33 | 0 | 1 | NA | 1 | 0 |
| Faunø Thrane | 2017 | 30 | 55 | 15 | 1 | NA | 30 | 0 |
| sideris | 2020 | 4 | 37 | 0 | 0.3 | NA | 4 | 0 |
| Rodrigues Silva | 2020 | 1 | 67 | 1 | 3 | 35 | 1 | 0 |
| Mcgowan | 2021 | 5 | 44.5 | 3 | 1.6 | 18.8 | 5 | 0 |
*, rounded to the nearest 0.5 years. NA, not available.
Odontogenic source infections were the most common (92, 57.1%), followed by tonsillar/peritonsillar (29, 18.0%) and postoperative/traumatic (27, 16.8%). The remaining cases were either from a cutaneous origin, pharyngolaryngeal origin, oesophageal, salivary, or originating from an extra-cervical region.
All cases were treated with a combination of empiric antibiotics and surgical debridement. The mean number of debridements per patient was 3 (range, 1–10). One hundred and thirteen cases included specific antibiotic data. The most common antibiotic used was penicillin (67, 59.3%) followed by metronidazole (57, 50.4%), clindamycin (54, 47.7%) and meropenum (46, 40%). In all cases with documented antibiotic regimens, multiple antibiotics were used simultaneously. The specific antibiotic regimen was not specified in 48 cases.
The mean number of organisms was 2.1 per patient. Where specific microbial growth was mentioned (grouped or ungrouped, 127 total cases), streptococcus species were most common either as a single organism or part of polymicrobial infection (76%), followed by Peptostreptococcus species (21%), Prevotella (20%) and Staphylococcus species (13%). There was no growth in 6% of these cases (Table 3).
Table 3
| Bacterial growth | Patients (n) | % total |
|---|---|---|
| Number of organisms (mean) | 2.1 | |
| No growth | 7 | 6% |
| Staphylococcus spp. | 16 | 13% |
| Streptococcus spp. | 96 | 76% |
| Eubacterium | 1 | 1% |
| Enterobacter | 6 | 5% |
| Enterococcus | 2 | 2% |
| Acinetobacter | 2 | 2% |
| Fusobacterium | 18 | 14% |
| Propinonibacter acnes | 2 | 2% |
| Lactobacillus spp. | 1 | 1% |
| Peptostreptococcus spp. | 27 | 21% |
| Serratia spp. | 1 | 1% |
| Eubacterium | 4 | 3% |
| Klebsiella spp. | 3 | 2% |
| Pseudomonas spp. | 5 | 4% |
| Proteus | 2 | 2% |
| E.coli | 8 | 6% |
| Candida | 8 | 6% |
| Bacteroides | 14 | 11% |
| Corynebacterium | 4 | 3% |
| Haemophilus | 3 | 2% |
| Veillonella | 2 | 2% |
| Clostridium spp. | 3 | 2% |
| Eikenella | 1 | 1% |
| Neisseria | 4 | 3% |
| Arcanobacterium | 1 | 1% |
| Prevotella | 26 | 20% |
Where ungrouped individual patient growth data was available (32 patients) 56% of cases were polymicrobial; 31.3% of patients had a purely aerobic culture, 12.5% of patients had purely anaerobic growth and 37.5% had both aerobic and anaerobic growth (mixed). There was no growth in 18.7% of ungrouped cases (Table 4). In these patients there were two mortalities which both occurred in patients with only aerobic bacterial growth; There were no mortalities in the mixed or anaerobic groups.
Table 4
| Microbial growth (genus) | Number of patients | % of patients |
|---|---|---|
| No growth | 6 | 19% |
| Polymicrobial | 18 | 56% |
| Staphylococcus | 4 | 13% |
| Streptococcus | 24 | 75% |
| Eubacterium | 1 | 3% |
| Enterobacter | 3 | 9% |
| Acinetobacter | 1 | 3% |
| Fusobacterium | 3 | 9% |
| Lactobacillus | 1 | 3% |
| Peptostreptococcus | 2 | 6% |
| Serratia spp. | 1 | 3% |
| Eubacterium | 1 | 3% |
| Klebsiella | 1 | 3% |
| Pseudomonas | 1 | 3% |
| Escherichia coli | 1 | 3% |
| Candida | 2 | 6% |
| Bacteroides | 8 | 25% |
| Haemophilus | 1 | 3% |
| Veillonella | 1 | 3% |
| Eikenella | 1 | 3% |
| Neisseria | 2 | 6% |
| Prevotella | 1 | 3% |
The mean number of complications per patient was 0.64. There were 39 documented cases of mediastinitis, 38 cases of multiorgan failure and 7 vascular complications. The mean number of days in hospital was 35.4 days. The mortality rate of patients treated with HBOT was 7.6%. Based on the Wilson score method, the pooled portion of mortality was 0.759 (95% CI: 0.0040–0.1281) and the pooled proportion of survivors was 0.9241 (95% CI: 0.8719–0.9560) (38).
Discussion
CNF (also known as necrotising soft tissue infection) is a rare condition, with a reported prevalence of approximately 2 per million population (4). It is a rapidly progressive condition with mortality rates as high as 13.36% (5). Regional spread (including mediastinitis), vascular compromise, airway compromise and systemic sepsis can lead to severe morbidity and death (39).
The mainstay of treatment of CNF includes early and aggressive surgical debridement with broad spectrum antibiotic therapy (25,26,39). The goal of debridement is to remove infected and necrotic debris, disrupting the cycle of ischaemic necrosis and allowing tissue healing and revascularization to take place. This is a well-established paradigm, however the role of HBOT in CNF remains unclear (5,9,35,39,40). HBOT is considered to be valuable in improving healing and preventing further tissue damage through the increase in the partial pressure of oxygen within infected soft tissues. This allows for continued cellular metabolism, increases fibroblast proliferation and maturation, promotes neutrophilic phagocytosis, inhibits the proliferation of anaerobic bacteria, and is thought to increase the protective immunological effects of oxygen free radicals (4,9,10,41-43).
The largest and most recent systematic review of CNF performed by Gunaratne et al. in 2018 reviewed mortality outcomes in 861 patients through 201 articles (5). Articles were isolated according to the same search criteria and involved the same database as our study (until 2017). We used the same search criteria as this study, with similar inclusion/exclusion criteria, however our study only included articles with patients who had undergone HBOT. In the Gunaratne study, the overall mortality rate was 13.36% in all patients with CNF, with or without the use of HBOT. In our study of only HBOT patients, the mortality rate of 7.6% is significantly less (difference in proportion =−0.058, Z=−2.13, P=0.017).
CNF can be caused by monomicrobial or polymicrobial infection (5,6). In accordance with the literature, cultures identified within our study were most commonly polymicrobial (60%, mean number of organisms 2.1) which may include opportunistic growth, particularly in the case of anaerobic organisms, representing a synergistic pattern of growth once ischaemic necrosis has already taken place (2,44). In the context of CNF, HBOT is thought to be valuable in its antibacterial effect against anaerobes, however there is limited clinical evidence of this. Within our study, 34 patients had clearly documented microbial growth, of these, there were only two mortalities, both of which occurred in the context of purely aerobic growth (patient 1: Streptococcus species alone, patient 2: Staphylococcus and Strepotococcus mixed growth). We theorise that the antimicrobial effects of HBOT may be more valuable in infections which include anaerobic bacteria and may be less efficacious in patients with purely anaerobic growth, however this result is limited by small case numbers. This finding will require further assessment in future studies with larger population groups.
The delivery of HBOT varies depending on institution and protocol, however the general principles of delivery are similar universally. It can be delivered through either a masks or a pressure chamber, with the use of 100% oxygen with controlled pressure of approximately 2–3 atm for 90–120 mins, ether 12 hourly or 24 hourly (35). Contraindications to hyperbaric oxygen include pneumothorax active pulmonary infections, pregnancy, claustrophobia, malignancy, active chemotherapy, seizure disorders and emphysema (9,45). Patients with severe medical comorbidities may be unable to be transferred to a HBOT facility or may have limited ability to tolerate the treatment environment. These patients may also require high level medical and surgical care which may be limited in an HBOT facility. Treatment may be complicated by pulmonary, middle ear, sinus or dental barotrauma, oxygen toxicity with seizures, decompression sickness or visual disturbance. In addition, hyperbaric oxygen is an expensive, and not a universally accessible therapy (9,35,40,45,46).
As a result of this systematic review, we suggest that the ideal management of CNF includes urgent airway and haemodynamic management, early surgical debridement of necrotic tissues (repeated as required until only healthy, bleeding tissue is present), empiric broad spectrum antibiotic therapy (followed by targeted therapy guided by intraoperative cultures) and the addition of HBOT when available. There is currently limited evidence to support any specific HBOT schedule and dosage, and this remains dependant on facility guidelines at present.
The findings of this systematic review suggest that there is an improvement in mortality in patients who have undergone HBOT for CNF, however the limitations of this study are acknowledged. Due to the infrequency of CNF and the limited availability of HBOT, there are no randomised trials reviewing this topic, and as such, data is limited to case studies and case series. Additionally, the criteria used to diagnose patients with CNF varies between studies, ranging from clinical, to radiologic, to histologic. In the majority of the included studies, the criteria used for diagnosis was not specified or unclear. Additionally, the heterogeneity of demographic data, severity of primary infection, comorbidities and treatment regimens made comparison between groups problematic. There were limited case series which presented individual patient data and outcomes clearly, and many were presented in a grouped format with grouped analysis, limiting the data available to perform a meta-analysis. It is also acknowledged that in treatment of CNF, antibiotic regimens and the administration of HBOT vary both geographically and chronologically. Of the 12 mortalities in our systematic review, 10 were from a single paper which acts as an outlier and limits the reliability of our analysis (33). In addition, the omission of HBOT in critically unwell patients is a confounding factor that is likely to impact the primary mortality outcome, which may skew the survival data in the favour of HBOT. We also recognize a likely publication bias when discussing mortality rate, however this bias may be negated as our mortality rate has been compared directly with the mortality rate of a similar systematic review with similar inclusion and exclusion criteria.
Conclusions
This study utilises the largest database of patients with CNF treated with HBOT in the literature to identify all relevant epidemiologic and aetiologic data, diagnostic and management characteristics and outcome factors in these patients. The mortality rate of 7.5% in HBOT patients is lower than the previously published rate of CNF in a comparative systematic review published by Gunaratne et al. in 2018. As a result, we recommend that HBOT may be a useful adjunct to urgent surgical debridement and antibiotic therapy in patients with CNF. This treatment may be less efficacious in aerobic infections. The implication of these findings may assist in the future clinical management of CNF, however further prospective research is required to identify the true benefit of treatment.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://www.theajo.com/article/view/10.21037/ajo-23-5/rc
Peer Review File: Available at https://www.theajo.com/article/view/10.21037/ajo-23-5/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure forms (available at https://www.theajo.com/article/view/10.21037/ajo-23-5/coif). FR serves as an unpaid editorial board member of Australian Journal of Otolaryngology. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study did not require ethics approval as no patient data was accessed and no patients contact was required.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hua J, Friedlander P. Cervical Necrotizing Fasciitis, Diagnosis and Treatment of a Rare Life-Threatening Infection. Ear Nose Throat J 2023;102:NP109-13. [Crossref] [PubMed]
- Krenk L, Nielsen HU, Christensen ME. Necrotizing fasciitis in the head and neck region: an analysis of standard treatment effectiveness. Eur Arch Otorhinolaryngol 2007;264:917-22. [Crossref] [PubMed]
- Lin C, Yeh FL, Lin JT, et al. Necrotizing fasciitis of the head and neck: an analysis of 47 cases. Plast Reconstr Surg 2001;107:1684-93. [Crossref] [PubMed]
- Wolf H, Rusan M, Lambertsen K, et al. Necrotizing fasciitis of the head and neck. Head Neck 2010;32:1592-6. [Crossref] [PubMed]
- Gunaratne DA, Tseros EA, Hasan Z, et al. Cervical necrotizing fasciitis: Systematic review and analysis of 1235 reported cases from the literature. Head Neck 2018;40:2094-102. [Crossref] [PubMed]
- Gore MR. Odontogenic necrotizing fasciitis: a systematic review of the literature. BMC Ear Nose Throat Disord 2018;18:14. [Crossref] [PubMed]
- Wong CH, Wang YS. The diagnosis of necrotizing fasciitis. Curr Opin Infect Dis 2005;18:101-6. [Crossref] [PubMed]
- Moon R. Undersea and Hyperbaric Medical Society Hyperbaric Oxygen Therapy Indications. 14th Edition North Palam Beach (FL): Best Publishing Company, 2019.
- Faunø Thrane J, Pikelis A, Ovesen T. Hyperbaric oxygen may only be optional in head and neck necrotizing fasciitis: a retrospective analysis of 43 cases and review of the literature. Infect Dis (Lond) 2017;49:792-8. [Crossref] [PubMed]
- Kang TS, Gorti GK, Quan SY, et al. Effect of hyperbaric oxygen on the growth factor profile of fibroblasts. Arch Facial Plast Surg 2004;6:31-5. [Crossref] [PubMed]
- Shah J. Hyperbaric oxygen therapy. J Am Col Certif Wound Spec 2010;2:9-13. [Crossref] [PubMed]
- Devaney B, Frawley G, Frawley L, et al. Necrotising soft tissue infections: the effect of hyperbaric oxygen on mortality. Anaesth Intensive Care 2015;43:685-92. [Crossref] [PubMed]
- Hajebrahimi S, Dalir Akbari N, Haji Kamanaj A, et al. Quality of the Systematic Reviews in Cochrane Gynecological Cancer Group and Their Understudied RCTs. J Obstet Gynaecol India 2022;72:346-51. [Crossref] [PubMed]
- Krespi YP, Lawson W, Blaugrund SM, et al. Massive necrotizing infections of the neck. Head Neck Surg 1981;3:475-81. [Crossref] [PubMed]
- Rapoport Y, Himelfarb MZ, Zikk D, et al. Cervical necrotizing fasciitis of odontogenic origin. Oral Surg Oral Med Oral Pathol 1991;72:15-8. [Crossref] [PubMed]
- Gaukroger MC. Cervicofacial necrotising fasciitis. Br J Oral Maxillofac Surg 1992;30:111-4. [Crossref] [PubMed]
- Jackson BS, Sproat JE. Necrotizing fasciitis of the head and neck with intrathoracic extension. J Otolaryngol 1995;24:60-3. [PubMed]
- De Backer T, Bossuyt M, Schoenaers J. Management of necrotizing fasciitis in the neck. J Craniomaxillofac Surg 1996;24:366-71. [Crossref] [PubMed]
- Ray AM, Bressler K, Davis RE, et al. Cervicofacial necrotizing fasciitis. A devastating complication of blepharoplasty. Arch Otolaryngol Head Neck Surg 1997;123:633-6. [Crossref] [PubMed]
- Burstin P, Gordon M. Bilateral peritonsillar and parapharyneal abscesses with cervial necrotizing fasciitis. Aust J Otolaryngol 1999;3:342.
- Dale RA, Hoffman DS, Crichton RO, et al. Necrotizing fasciitis of the head and neck: review of the literature and report of a case. Spec Care Dentist 1999;19:267-74. [Crossref] [PubMed]
- Francque SM, Van Laer C, Struyf N, et al. Perforating oesophageal carcinoma presenting as necrotizing fasciitis of the neck. Eur J Gastroenterol Hepatol 2001;13:1261-4. [Crossref] [PubMed]
- Ulubil SA, Iseri M, Ozturk M, et al. Cervical necrotizing fasciitis. J Otolaryngol Head Neck Surg 2009;38:E23-8. [PubMed]
- Treasure T, Hughes W, Bennett J. Cervical necrotizing fasciitis originating with a periapical infection. J Am Dent Assoc 2010;141:861-6. [Crossref] [PubMed]
- Chunduri NS, Madasu K, Tammannavar PS, et al. Necrotising fasciitis of odontogenic origin. BMJ Case Rep 2013;2013:bcr2012008506. [Crossref] [PubMed]
- Inan CH, Yener HM, Yilmaz M, et al. Cervical Necrotizing Fasciitis of Odontogenic Origin and Hyperbaric Oxygen Therapy. J Craniofac Surg 2017;28:e691-2. [Crossref] [PubMed]
- Silva VAR, Almeida AS, Lavinsky J, et al. Thorax necrotizing fasciitis following Bezold's abscess. Clin Case Rep 2020;8:2848-51. [Crossref] [PubMed]
- Djupesland PG. Necrotizing fascitis of the head and neck--report of three cases and review of the literature. Acta Otolaryngol Suppl 2000;543:186-9. [Crossref] [PubMed]
- Bayetto K, Cheng A, Sambrook P. Necrotizing fasciitis as a complication of odontogenic infection: a review of management and case series. Aust Dent J 2017;62:317-22. [Crossref] [PubMed]
- Cortese A, Pantaleo G, Borri A, et al. Necrotizing odontogenic fasciitis of head and neck extending to anterior mediastinum in elderly patients: innovative treatment with a review of the literature. Aging Clin Exp Res 2017;29:159-65. [Crossref] [PubMed]
- Maisel RH, Karlen R. Cervical necrotizing fasciitis. Laryngoscope 1994;104:795-8. [Crossref] [PubMed]
- Langford FP, Moon RE, Stolp BW, et al. Treatment of cervical necrotizing fasciitis with hyperbaric oxygen therapy. Otolaryngol Head Neck Surg 1995;112:274-8. [Crossref] [PubMed]
- Mathieu D, Neviere R, Teillon C, et al. Cervical necrotizing fasciitis: clinical manifestations and management. Clin Infect Dis 1995;21:51-6. [Crossref] [PubMed]
- Whitesides L, Cotto-Cumba C, Myers RA. Cervical necrotizing fasciitis of odontogenic origin: a case report and review of 12 cases. J Oral Maxillofac Surg 2000;58:144-51; discussion 152. [Crossref] [PubMed]
- Flanagan CE, Daramola OO, Maisel RH, et al. Surgical debridement and adjunctive hyperbaric oxygen in cervical necrotizing fasciitis. Otolaryngol Head Neck Surg 2009;140:730-4. [Crossref] [PubMed]
- Sideris G, Nikolopoulos T, Delides A. Cervical necrotizing fasciitis affects only immunocompromized patients? Diagnostic challenges, treatment outcomes and clinical management of eleven immunocompetent adult patients with a still fatal disease. Am J Otolaryngol 2020;41:102613. [Crossref] [PubMed]
- McGowan S, Leet J, Christensen L, et al. Hyperbaric Oxygen as Adjunctive Therapeutic in Management of Craniocervical Necrotizing Fasciitis: Case Series and Treatment Protocol. J Oral Maxillofac Surg 2021;79:e91-2. [Crossref]
- Wallis S. Binomial Confidence Intervals and Contingency Tests: Mathematical Fundamentals and the Evaluation of Alternative Methods. J Quant Linguist 2013;20:178-208. [Crossref]
- Velhonoja J, Lääveri M, Soukka T, et al. Deep neck space infections: an upward trend and changing characteristics. Eur Arch Otorhinolaryngol 2020;277:863-72. [Crossref] [PubMed]
- Shaw JJ, Psoinos C, Emhoff TA, et al. Not just full of hot air: hyperbaric oxygen therapy increases survival in cases of necrotizing soft tissue infections. Surg Infect (Larchmt) 2014;15:328-35. [Crossref] [PubMed]
- Knighton DR, Fiegel VD, Halverson T, et al. Oxygen as an antibiotic. The effect of inspired oxygen on bacterial clearance. Arch Surg 1990;125:97-100. [Crossref] [PubMed]
- Korhonen K. Hyperbaric oxygen therapy in acute necrotizing infections. With a special reference to the effects on tissue gas tensions. Ann Chir Gynaecol 2000;89:7-36. [PubMed]
- Jones SR, Carpin KM, Woodward SM, et al. Hyperbaric oxygen inhibits ischemia-reperfusion-induced neutrophil CD18 polarization by a nitric oxide mechanism. Plast Reconstr Surg 2010;126:403-11. [Crossref] [PubMed]
- Bryant AE, Bayer CR, Chen RY, et al. Vascular dysfunction and ischemic destruction of tissue in Streptococcus pyogenes infection: the role of streptolysin O-induced platelet/neutrophil complexes. J Infect Dis 2005;192:1014-22. [Crossref] [PubMed]
- Ambiru S, Furuyama N, Aono M, et al. Analysis of risk factors associated with complications of hyperbaric oxygen therapy. J Crit Care 2008;23:295-300. [Crossref] [PubMed]
- Plafki C, Peters P, Almeling M, et al. Complications and side effects of hyperbaric oxygen therapy. Aviat Space Environ Med 2000;71:119-24. [PubMed]
Cite this article as: Tseros EA, Reddy R, Ho J, Gunaratne DA, Venkatesha V, Riffat F. Improvement in mortality with hyperbaric oxygen therapy in cervical necrotising fasciitis: a systematic review of the literature. Aust J Otolaryngol 2023;6:8.


