
Surgical outcomes in a retrospective cohort of adult patients with severe obstructive sleep apnea
Introduction
Obstructive sleep apnea (OSA) is a disorder of altered breathing during sleep (1). It is undiagnosed in a significant proportion of the population and affects almost one billion people worldwide (1,2). Characteristic periods of apnea and hypopnea occur due to repetitive collapse of the upper airways during sleep. OSA can increase cardiovascular risk (2), impair neurological function (3), increase daytime somnolence (4), increase the incidence of motor vehicle accidents (5), and reduce quality of life (5).
The treatment approach for OSA factors in patient goals and motivations and targets a reduction in daytime sleepiness, improvement in quality of life, and improvement in objective polysomnographic parameters of disease severity (6). Two objective polysomnographic parameters include the apnea-hypopnea index (AHI) and nocturnal oxygen desaturation index (ODI).
Continuous positive airway pressure (CPAP) is the mainstay of OSA treatment and improves daytime sleepiness, neurocognitive function, and hypertension (7). Optimal usage may reduce overall and cardiovascular mortality (7) although the evidence for this is uncertain (8).
Adequate CPAP usage is a major limiting factor in OSA treatment. When adherence to CPAP is defined as four hours or more per night, between 46% and 83% of patients are reported as non-adherent (9). Subtherapeutic CPAP use has been associated with continued exposure to sleep-disordered breathing and persistent symptomatology (10). Pre-treatment disease severity interacts with CPAP adherence to determine residual disease severity, and it has been argued that this concept should help define adequate CPAP utilization (11).
Patients who demonstrate they cannot adequately meet CPAP adherence standards should be considered for alternative therapy, including positional treatment, oral appliance devices, and upper airway surgery (9). Surgical treatment may be effective in patients with specific anatomical characteristics, for example, low body mass index (BMI), smaller tongue and large tonsils. Surgical treatment for OSA is associated with improved quality of life and reduced risk of cardiovascular disease compared with untreated or ineffectively treated OSA (12), with observational studies suggesting a mortality benefit (13). There is a dose-response relationship between disease severity and extent of cardiovascular risk, and those with untreated very severe OSA are likely to be at higher risk of morbidity and mortality (2,14). There are few studies however that investigate outcomes after surgical treatment in patients with very severe OSA.
This study investigated the changes in OSA severity after multilevel upper airway surgery in selected patients with very severe OSA who were non-adherent to CPAP. Very severe OSA has been variably defined in the literature, with cut-off AHIs ranging between 40 to 70 (15-18). In this study, very severe OSA was defined as having an AHI ≥50 or a 3% overnight desaturation index (ODI) >40. We present this article in accordance with the STROCSS reporting checklist (available at https://www.theajo.com/article/view/10.21037/ajo-20-80/rc).
Methods
Setting and design
This was a retrospective single cohort study of patients with very severe OSA undergoing multilevel upper airway surgery between January 1st 2009, to December 31st 2018. Surgeries were performed at Wollongong Hospital and Wollongong Private Hospital in New South Wales, Australia.
Patient eligibility
Full inclusion and exclusion criteria are found in Appendix 1. Eligible patients were aged 18 to 70 years and recruited from an outpatient sleep clinic. Patients were diagnosed with very severe OSA (defined by AHI ≥50 and 3% ODI >40). The AHI is the combined average of the number of apneas and hypopneas that occur per hour during sleep. All patients had at least level two polysomnography at baseline. Polysomnography is divided into four tiers. Level one is the most comprehensive sleep study, with monitoring of neurological, muscular, and respiratory parameters. Level two is similar to level one but can be done as an outpatient. Level three focuses only on respiratory parameters, and level four usually only monitors oxygen saturation. All patients failed CPAP treatment for various reasons, including claustrophobia, inability to tolerate a mask, bloating, numbness, and interference with intimacy. This was despite the involvement of a sleep physician to discuss and review mask tolerance.
Participants were assessed preoperatively in a combined multidisciplinary setting, including a sleep physician and otolaryngologist.
Intervention and outcome measures
All patients underwent single or multiple-stage multilevel airway reconstructive surgery by a single sleep surgery fellowship-trained otolaryngologist (SM) with over five-year’s experience in the field of sleep surgery. Patient demographic and relevant clinical information such as age, sex, BMI (kg/m2), tonsil grade and Friedman stage were collected (19). Postoperatively, patients were encouraged to lose weight or retrial positioning devices or CPAP to optimize results.
Flexible nasendoscopy with awake dynamic manoeuvres was used to identify the planes and levels of collapse within the pharynx and exclude any other anatomical and pathological causes of obstruction. Staged surgical protocols were followed, consistent with previously published evidence-based guidelines and trial protocols (20,21). Nasal function was optimized prior to enrolment in the study to ensure an adequate nasal airway, and select patients had concurrent turbinate surgery if required. Phase 1 surgery in every enrolled patient included but was not exclusive to: Australian modified uvulopalatopharyngoplasty (“modified UPPP”) radiofrequency-in-saline tongue channelling, with concurrent Palatine Tonsillectomy if tonsils were present (22). Concurrent Lingual Tonsillectomy was performed if grade 3 or 4 lingual tonsils were present and contributing to upper airway obstruction (23). Phase 2 surgical options were again guided by awake endoscopic determination of the contributory level of airway obstruction. These included midline glossectomy if retrolingual airway obstruction was occurring secondary to macroglossia (24) and Trans-Palatal Advancement Pharyngoplasty (“TPAP”) if airway obstruction was occurring secondary to uncontrolled retropalatal airway collapse (25,26). Initial postoperative care was attended for 1–2 nights in a high dependency setting for close observation of respiratory and upper airway status, although invasive airway management was not required for any patients postoperatively. Postoperative recovery was otherwise completed in an ambulatory setting.
Full-night attended polysomnography was performed on average five months postoperatively. The primary outcome was the change in AHI (events/hour). Secondary outcomes included the achievement of “surgical success” which was defined as a postoperative AHI <20 and a 50% reduction in AHI (27). Other secondary outcomes included change in 3% ODI (events/hour), lowest measured oxygen saturations (oxygen saturation %), snoring severity scale through the bedpartner questionnaire (28), and Epworth sleepiness scale. We also noted adverse events after surgery. Serious adverse events were defined as resulting in patient death; life-threatening illness or injury; permanent impairment of body structure or function; in-patient hospitalization (>24 h) or prolongation of existing hospitalization; medical or surgical intervention to prevent permanent impairment to body structure or body function. Standard of care emergency protocols locally dictated that patients with postoperative bleeding following pharyngeal surgery be admitted for observation, even if minor. Admission for postoperative bleeding was classified as a serious adverse event only if surgical intervention was required.
Statistical analysis
Paired two-sided t-tests were used to compare continuous variables of normal distribution, Wilcoxon signed-rank test was used to compare continuous variables of non-normal distribution, and chi-squared tests were used for categorical variables. Significant was set at the threshold is P<0.05. Statistical analysis was done through SPSS statistics version 21 (IBM, Chicago, USA).
Ethics and reporting guidelines.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The University of Queensland Office of Research ethics deemed this study exempt from ethics review under the National Statement on Ethical Conduct in Human Research and University of Queensland policy (Clearance Number 2020001667). Individual consent for this retrospective analysis was waived (29).
Results
A total of 39 patients with very severe OSA were included in the study, of which 31 (79%) were male, the mean age was 41 years (standard deviation 13), and the mean BMI was 31 kg/m2 (standard deviation 5). Most patients (24, 62%) had a tonsil grade of 3–4 (Table 1). Twenty-five patients (64%) had a Friedman stage of 1 or 2, and the remaining 14 had a Friedman stage of 3 or 4.
Table 1
| Characteristic | Number |
|---|---|
| Age at procedure (years), mean ± SD | 41±13 |
| Sex, n [%] | |
| Male | 31 [79] |
| Female | 8 [21] |
| Tonsil grade, n [%] | |
| 0–2 | 15 [38] |
| 3–4 | 24 [62] |
| Friedman stage, n [%] | |
| 1–2 | 25 [64] |
| 3–4 | 14 [36] |
SD, standard deviation.
All patient underwent step-wise surgical treatment in various combinations (Table 2). Postoperatively one patient retrialed CPAP usage.
Table 2
| Interventions 1 and 2 | Intervention 3 | Intervention 4 | Intervention 5 | No. of patients [%] |
|---|---|---|---|---|
| Modified uvulopalatopharyngoplasty and radiofrequency-in-saline tongue channelling with or without tonsillectomy* | – | – | – | 21 [54] |
| Turbinate reduction | – | – | 2 [5] | |
| Trans-palatal advancement pharyngoplasty | – | – | 1 [3] | |
| Midline glossectomy | – | – | 1 [3] | |
| Midline glossectomy | Trans-palatal advancement pharyngoplasty | – | 6 [15] | |
| Lingual tonsillectomy | – | – | 4 [10] | |
| Lingual tonsillectomy | Trans-palatal advancement pharyngoplasty | – | 2 [5] | |
| Lingual tonsillectomy | Midline glossectomy | Trans-palatal advancement pharyngoplasty | 2 [5] |
*, all 39 patients underwent modified uvulopalatopharyngoplasty and radiofrequency-in-saline tongue channelling with or without tonsillectomy.
Mean preoperative AHI was 69 compared with postoperative AHI of 14 (P<0.01). Twenty-nine patients (74%) achieved surgical success (an overall AHI <20 and a greater than 50% reduction). The 3% ODI reduced from 54 events per hour to 12 (P<0.01), and the lowest recorded oxygen saturation level increased from a mean of 73% to 81% (P<0.01) (Table 3).
Table 3
| Characteristic | Preoperative | Postoperative | Mean difference (95% confidence interval) | P value |
|---|---|---|---|---|
| BMI (kg/m2), mean ± SD | 31±5 | 29±5 | 1 [0.5–2] | <0.01 |
| AHI (events/hour), mean ± SD | 69±16 | 14±11 | 55 [48–62] | <0.01 |
| 3% ODI (events/hour), mean ± SD | 54±15 | 12±16 | 41 [32–50] | <0.01 |
| Lowest oxygen saturations (%), mean ± SD | 73±10 | 81±20 | −9 [−17 to −1] | 0.02 |
| Snoring severity scale (/9), median [range] | 8 [4–9] | 0.5 [0–7] | – | <0.01 |
| Epworth sleepiness scale (/24), median [range] | 12 [1–22] | 4 [0–14] | – | <0.01 |
*, variables without full data from 39 patients: snoring severity (26 patients included); ODI (30 patients included); lowest oxygen saturations (38 patients included); BMI (38 patients included); Epworth sleepiness scale (35 patients included). BMI, body mass index; AHI, apnea-hypopnea index; ODI, oxygen desaturation index; SD, standard deviation.
Symptomatic outcomes also showed improvement, with snoring severity improving from a median of 8 to 0.5 (P<0.01) and reduction on the Epworth sleepiness scale from 12 preoperatively to 4 postoperatively (P<0.01) (Table 3).
Adverse events were all classified as minor and were known complications following multilevel airway surgery. Four patients suffered postoperative hemorrhage requiring admission and observation, but none required return to the operating theatre for bleeding control. Two patients described a transient foreign body sensation in the pharynx, and one patient had postoperative transient velopharyngeal incompetence. One patient had a transient oronasal fistula following trans-palatal advancement pharyngoplasty (30), which healed with conservative therapy.
Discussion
This study demonstrates that upper airway surgery can have significant and clinically meaningful improvements in patients with very severe OSA who are otherwise sub-optimally treated with CPAP. This contradicts the theory that airway surgery is more effective in milder disease (31). A significant improvement in objective measures of OSA was found, including the primary outcome AHI, as well as secondary outcomes of 3% ODI and lowest measured oxygen saturation. There was a considerable reduction in mean AHI postoperative, from 69 to 14. This substantial improvement in OSA severity may be attributed to careful preoperative patient selection, application of a phased multilevel paradigm, and utilization of an interdisciplinary approach to OSA. We also noted a significant improvement in ODI, which may be a useful surrogate of cardiovascular risk and overall mortality (32). Self-reported subjective measures (including Epworth sleepiness and snoring severity) also showed clinically relevant improvements.
The results of this study correlate with a growing body of evidence that demonstrates significant improvements in OSA disease severity and symptoms following airway surgery (12,33-35). The SAMS trial randomized 102 participants with moderate or severe OSA and found a significant reduction in AHI (−17.6 events/hour) and Epworth sleepiness scale (−6.7) six months post multilevel surgery (modified UPPP and minimally invasive tongue volume reduction) compared to medical management (12). Similarly, Sommer et al. illustrated in a study of 42 randomized participants a significant drop in mean AHI post-tonsillectomy and UPPP at three months with concurrent improvement in daytime sleepiness between treatment and control groups (34). Finally, the SKUP3 trial demonstrated in 65 participants a significant reduction in AHI post-UPPP compared to control at six months, with a 60% reduction of AHI in the surgical group and 11% in control (33). Improvements in ODI were also seen in both the SAMS and the SKUP3 trial, as were improvements in Epworth sleepiness scale.
Observational data has also shown improvements in AHI and measures of sleepiness with upper airway surgery (36). Various surgical methods (including traditional UPPP, palate surgery, maxillo-mandibular advancement, and upper airway radiofrequency-in-saline tongue channelling) have decreased AHI (36). Finally, bilateral tonsillectomy in isolation can achieve a mean AHI reduction of 65% in patients with at least grade 2 tonsils (37). Some observational studies suggest that surgery may reduce cardiovascular-related morbidity and mortality (14,38).
Multilevel airway collapse is present in most patients with OSA (39); hence multilevel and multi-phase surgery has been advocated for the surgical treatment of OSA and was employed in our study (20). All of the patients in this study underwent a combination of a contemporary modification of UPPP with radiofrequency-in-saline treatment of the tongue (22). Patients with severe OSA may be less likely to respond to single staged interventions, suggesting that the “failure” of surgery in severe OSA may reflect inadequate volume of surgery as opposed to intrinsic inadequacies in surgery (40).
Despite the efficacy of CPAP in treating upper airway obstruction, real-life effectiveness is limited by adherence (9). Many patients cannot reach a level of adherence where they receive symptomatic benefit or see improved cardiovascular parameters (9). Using data and calculations from Sutherland et al. (41) a patient with a baseline AHI of 60 would require over 6 hours of CPAP usage per night to achieve a residual AHI of 16 (Figure 1). This is nearly double the mean usage seen in the largest randomized trial on cardiovascular outcomes with CPAP and represents a required usage rate in excess of what is published in the literature (11,42).
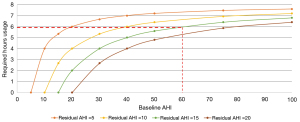
It has been argued that pre-treatment OSA severity and therapeutic device adherence should be considered together to determine if a patient is adequately treated—concepts which have been variably named the “SARAH Index” (Sleep Adjusted Residual AHI) (41) and “mean AHI on CPAP” (43). In individuals with symptomatic OSA who are poorly or non-adherent to CPAP, the SARAH Index may be lower following upper airway surgery than continuing to pursue device therapy (43,44).
There are several strengths of this study. The novel idea of selecting and analyzing the results of patients with very severe OSA has rarely been done in the past (16). The study of objective outcomes and patient-reported measures demonstrated improvements across these broad outcomes.
Limitations are in the retrospective nature of this study, the small numbers, and the lack of blinding for investigators. The results of this study may not be broadly applicable to patients with severe OSA as patient selection was dependent on strict inclusion and exclusion criteria, with specific improvable anatomical traits as assessed by an experienced sleep surgeon. We note a slight decrease in BMI after surgery and standard medical advice regarding weight loss, and that improvement in OSA parameters are also related to this (45). However, the considerable change in AHI seen in this study is well in excess of the expected improvement seen in previous weight loss studies for OSA and cannot only be explained by a two-point reduction in BMI (46). Furthermore, one patient retrialed CPAP. While this most probably improved their sleep apnea parameters in adjunct to the surgical treatment, their results did not deviate greatly from the other participants.
Conclusions
Surgery may be a valuable treatment option for very severe OSA in carefully selected patients, who should be identified and referred to a sleep surgeon. This data shows that excellent AHI and patient-centred outcomes may be achieved with salvage multilevel upper-airway surgery. Further, larger prospective studies are needed, and an increase in training in sleep surgery is needed.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROCSS reporting checklist. Available at https://www.theajo.com/article/view/10.21037/ajo-20-80/rc
Data Sharing Statement: Available at https://www.theajo.com/article/view/10.21037/ajo-20-80/dss
Peer Review File: Available at https://www.theajo.com/article/view/10.21037/ajo-20-80/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://www.theajo.com/article/view/10.21037/ajo-20-80/coif). SM reports no conflict of interest specific to this study but received funding for SAMS upper airway surgery clinical trial through the National Health and Medical Research Council’s (NHMRC) and does consultancy for Genio-Nyxoah, CN XII and INvicta, with travel support from Inspire. He also serves as an unpaid editorial board member of Australian Journal of Otolaryngology. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The University of Queensland Office of Research ethics deemed this study exempt from ethics review under the National Statement on Ethical Conduct in Human Research and University of Queensland policy (Clearance Number 2020001667). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med 2019;7:687-98. [Crossref] [PubMed]
- Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep 2008;31:1071-8. [PubMed]
- Leng Y, McEvoy CT, Allen IE, et al. Association of Sleep-Disordered Breathing With Cognitive Function and Risk of Cognitive Impairment: A Systematic Review and Meta-analysis. JAMA Neurol 2017;74:1237-45. [Crossref] [PubMed]
- Lal C, Weaver TE, Bae CJ, et al. Excessive Daytime Sleepiness in Obstructive Sleep Apnea. Mechanisms and Clinical Management. Ann Am Thorac Soc 2021;18:757-68. [Crossref] [PubMed]
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 2002;165:1217-39. [Crossref] [PubMed]
- Sarkissian L, Kitipornchai L, Cistulli P, et al. An update on the current management of adult obstructive sleep apnoea. Aust J Gen Pract 2019;48:182-6. [Crossref] [PubMed]
- Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005;365:1046-53. [Crossref] [PubMed]
- McEvoy RD, Antic NA, Heeley E, et al. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. N Engl J Med 2016;375:919-31. [Crossref] [PubMed]
- Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc 2008;5:173-8. [Crossref] [PubMed]
- Kohler M, Stoewhas AC, Ayers L, et al. Effects of continuous positive airway pressure therapy withdrawal in patients with obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med 2011;184:1192-9. [Crossref] [PubMed]
- Bakker JP, Weaver TE, Parthasarathy S, et al. Adherence to CPAP: What Should We Be Aiming For, and How Can We Get There? Chest 2019;155:1272-87. [Crossref] [PubMed]
- MacKay S, Carney AS, Catcheside PG, et al. Effect of Multilevel Upper Airway Surgery vs Medical Management on the Apnea-Hypopnea Index and Patient-Reported Daytime Sleepiness Among Patients With Moderate or Severe Obstructive Sleep Apnea: The SAMS Randomized Clinical Trial. JAMA 2020;324:1168-79. [Crossref] [PubMed]
- Weaver EM, Maynard C, Yueh B. Survival of veterans with sleep apnea: continuous positive airway pressure versus surgery. Otolaryngol Head Neck Surg 2004;130:659-65. [Crossref] [PubMed]
- Peker Y, Carlson J, Hedner J. Increased incidence of coronary artery disease in sleep apnoea: a long-term follow-up. Eur Respir J 2006;28:596-602. [Crossref] [PubMed]
- Hamaoka T, Murai H, Takata S, et al. Different prognosis between severe and very severe obstructive sleep apnea patients; Five year outcomes. J Cardiol 2020;76:573-9. [Crossref] [PubMed]
- Huang EI, Lin YC, Huang SY, et al. Shifting and reducing breathing disturbance in patients with very severe obstructive sleep apnea by modified Z-palatoplasty with one-layer closure in one-stage multilevel surgery. Sci Rep 2021;11:8472. [Crossref] [PubMed]
- Cillo JE Jr, Finn R, Dasheiff RM. Combined open rhinoplasty with spreader grafts and laser-assisted uvuloplasty for sleep-disordered breathing: long-term subjective outcomes. J Oral Maxillofac Surg 2006;64:1241-7. [Crossref] [PubMed]
- Varghese MJ, Sharma G, Shukla G, et al. Longitudinal ventricular systolic dysfunction in patients with very severe obstructive sleep apnea: A case control study using speckle tracking imaging. Indian Heart J 2017;69:305-10. [Crossref] [PubMed]
- Friedman M, Salapatas AM, Bonzelaar LB. Updated Friedman Staging System for Obstructive Sleep Apnea. Adv Otorhinolaryngol 2017;80:41-8. [Crossref] [PubMed]
- Carney AS, Antic NA, Catcheside PG, et al. Sleep Apnea Multilevel Surgery (SAMS) trial protocol: a multicenter randomized clinical trial of upper airway surgery for patients with obstructive sleep apnea who have failed continuous positive airway pressure. Sleep 2019;42:zsz056. [Crossref] [PubMed]
- MacKay SG, Lewis R, McEvoy D, et al. Surgical management of obstructive sleep apnoea: A position statement of the Australasian Sleep Association. Respirology 2020;25:1292-308. [Crossref] [PubMed]
- MacKay SG, Carney AS, Woods C, et al. Modified uvulopalatopharyngoplasty and coblation channeling of the tongue for obstructive sleep apnea: a multi-centre Australian trial. J Clin Sleep Med 2013;9:117-24. [Crossref] [PubMed]
- Robinson S, Ettema SL, Brusky L, et al. Lingual tonsillectomy using bipolar radiofrequency plasma excision. Otolaryngol Head Neck Surg 2006;134:328-30. [Crossref] [PubMed]
- MacKay SG, Jefferson N, Grundy L, et al. Coblation-assisted Lewis and MacKay operation (CobLAMO): new technique for tongue reduction in sleep apnoea surgery. J Laryngol Otol 2013;127:1222-5. [Crossref] [PubMed]
- Woodson BT, Toohill RJ. Transpalatal advancement pharyngoplasty for obstructive sleep apnea. Laryngoscope 1993;103:269-76. [Crossref] [PubMed]
- Volner K, Dunn B, Chang ET, et al. Transpalatal advancement pharyngoplasty for obstructive sleep apnea: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol 2017;274:1197-203. [Crossref] [PubMed]
- Sher AE, Schechtman KB, Piccirillo JF. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep 1996;19:156-77. [Crossref] [PubMed]
- Lim PV, Curry AR. A new method for evaluating and reporting the severity of snoring. J Laryngol Otol 1999;113:336-40. [Crossref] [PubMed]
- Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med 2007;4:e297. [Crossref] [PubMed]
- Chan L, Kitpornchai L, Mackay S. Causative Factors for Complications in Transpalatal Advancement. Ann Otol Rhinol Laryngol 2020;129:18-22. [Crossref] [PubMed]
- Friedman M, Vidyasagar R, Bliznikas D, et al. Does severity of obstructive sleep apnea/hypopnea syndrome predict uvulopalatopharyngoplasty outcome? Laryngoscope 2005;115:2109-13. [Crossref] [PubMed]
- Temirbekov D, Güneş S, Yazıcı ZM, et al. The Ignored Parameter in the Diagnosis of Obstructive Sleep Apnea Syndrome: The Oxygen Desaturation Index. Turk Arch Otorhinolaryngol 2018;56:1-6. [Crossref] [PubMed]
- Browaldh N, Nerfeldt P, Lysdahl M, et al. SKUP3 randomised controlled trial: polysomnographic results after uvulopalatopharyngoplasty in selected patients with obstructive sleep apnoea. Thorax 2013;68:846-53. [Crossref] [PubMed]
- Sommer UJ, Heiser C, Gahleitner C, et al. Tonsillectomy with Uvulopalatopharyngoplasty in Obstructive Sleep Apnea. Dtsch Arztebl Int 2016;113:1-8. [Crossref] [PubMed]
- Browaldh N, Bring J, Friberg D. SKUP(3) RCT; continuous study: Changes in sleepiness and quality of life after modified UPPP. Laryngoscope 2016;126:1484-91. [Crossref] [PubMed]
- Caples SM, Rowley JA, Prinsell JR, et al. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep 2010;33:1396-407. [Crossref] [PubMed]
- Camacho M, Li D, Kawai M, et al. Tonsillectomy for adult obstructive sleep apnea: A systematic review and meta-analysis. Laryngoscope 2016;126:2176-86. [Crossref] [PubMed]
- Marti S, Sampol G, Muñoz X, et al. Mortality in severe sleep apnoea/hypopnoea syndrome patients: impact of treatment. Eur Respir J 2002;20:1511-8. [Crossref] [PubMed]
- Fujita S, Conway W, Zorick F, et al. Surgical Correction of Anatomic Abnormalities in Obstructive Sleep Apnea Syndrome: Uvulopalatopharyngoplasty. Otolaryngology–Head and Neck Surgery 1981;89:923-34. [Crossref] [PubMed]
- Riley RW, Powell NB, Guilleminault C. Obstructive sleep apnea syndrome: a review of 306 consecutively treated surgical patients. Otolaryngol Head Neck Surg 1993;108:117-25. [Crossref] [PubMed]
- Sutherland K, Phillips CL, Cistulli PA. Efficacy versus Effectiveness in the Treatment of Obstructive Sleep Apnea: CPAP and Oral Appliances. J Dent Sleep Med 2015;2:175-81. [Crossref]
- Chai-Coetzer CL, Luo YM, Antic NA, et al. Predictors of long-term adherence to continuous positive airway pressure therapy in patients with obstructive sleep apnea and cardiovascular disease in the SAVE study. Sleep 2013;36:1929-37. [Crossref] [PubMed]
- Ravesloot MJ, de Vries N, Stuck BA. Treatment adherence should be taken into account when reporting treatment outcomes in obstructive sleep apnea. Laryngoscope 2014;124:344-5. [Crossref] [PubMed]
- Sutherland K, Vanderveken OM, Tsuda H, et al. Oral appliance treatment for obstructive sleep apnea: an update. J Clin Sleep Med 2014;10:215-27. [Crossref] [PubMed]
- Peppard PE, Young T, Palta M, et al. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA 2000;284:3015-21. [Crossref] [PubMed]
- Mitchell LJ, Davidson ZE, Bonham M, et al. Weight loss from lifestyle interventions and severity of sleep apnoea: a systematic review and meta-analysis. Sleep Med 2014;15:1173-83. [Crossref] [PubMed]
Cite this article as: Huang JD, Shao EX, MacKay S, Kitipornchai L. Surgical outcomes in a retrospective cohort of adult patients with severe obstructive sleep apnea. Aust J Otolaryngol 2023;6:11.


