
Facial canal dehiscence in cholesteatoma and co-existing surgical findings: a systematic review and meta-analysis
Introduction
The facial nerve is an important anatomic structure in middle ear surgery and travels within the Z-shaped bony facial canal between the internal acoustic meatus and stylomastoid foramen. Facial canal dehiscence (FCD) is defined as erosion of or discontinuity in the bony structure of the facial canal, allowing for communication between the facial nerve and the middle ear cavity. It may be present in the normal population as a congenital anatomic variant due to incomplete ossification during intra-uterine life and early childhood (1). Pathological dehiscence can be secondary to inflammatory, infectious, or neoplastic processes that affect the middle ear, as well as previous trauma or surgical instrumentation. The tympanic segment of the facial canal is the most common site of FCD, with dehiscence generally occurring in proximity to the oval window (2). The clinical significance of FCD in cholesteatoma is its association with more extensive disease and increased incidence of further destructive findings intra-operatively—this includes labyrinthine fistula and erosion of the ossicular chain, scutum, external auditory canal, and tegmen tympani (3). The importance to the surgeon is that dehiscence of the facial canal increases risk of iatrogenic injury to the facial nerve during middle ear surgery as it lacks a protective bony covering. The gold standard for clinical diagnosis of FCD is intraoperative examination using a microscope. Pre-operative imaging with dedicated computed tomography (CT) petrous temporal bones is useful in predicting FCD and assists surgical planning. It facilitates assessment of the course of the intratemporal facial nerve and, in cases where FCD is not obvious, aids detection of other cautionary erosive findings that should raise suspicion for dehiscence being encountered intraoperatively (4). The objective of this systematic review and meta-analysis is to determine the pooled prevalence of FCD in patients who underwent cholesteatoma surgery and discuss coexisting surgical findings that correlate with FCD. We present this article in accordance with the PRISMA reporting checklist (available at https://www.theajo.com/article/view/10.21037/ajo-23-1/rc) (Figure 1).

Methods
Study design and search strategy
A systematic review and meta-analysis were performed in accordance with the PRISMA guidelines. An a priori study protocol was not lodged. A literature review was performed on 25th October 2021 using the following databases, including studies from their earliest date of cataloguing: PubMed (which includes MEDLINE data), MEDLINE, Embase, and Cochrane Library. Two main search domains were used, which were combined with the Boolean operator “And”, whilst search terms contained within each domain were combined with the Boolean operator “Or”. The keywords within the first search domain were “cholesteatoma”, and within the second search domain were “facial nerve”, “facial canal”, “fallopian canal”, and “dehiscence”. The reference lists of all included articles were searched by the authors to identify further articles that met the inclusion criteria. The Google Scholar database was utilised to supplement the literature review.
Approval from the ethics institutional review board was not required for this study as it is a systematic review and meta-analysis of published literature and it does not require collection of patient data.
Study selection and eligibility criteria
Two authors (S Ananthapadmanabhan, G Budiono) independently assessed the titles, abstracts, and full-text articles of potentially eligible studies using pre-determined inclusion criteria. Studies that reported the incidence of FCD in patients with cholesteatoma were included, in which dehiscence was diagnosed by intraoperative examination. All age groups including studies reporting on adult, paediatric, or combined populations were included. Both prospective and retrospective studies were included. Exclusion criteria included case reports, unpublished studies, studies without surgical confirmation of FCD, and studies with mixed pathology data sets in which the cholesteatoma cohort could not be isolated. Studies that focussed solely on medially invasive and extensive cholesteatomas or cholesteatomas with intracranial complications were excluded as the incidence of FCD would be grossly inflated. The search was limited to the English language and articles published between 1981–2021.
Titles and abstracts were first independently assessed by the authors (S Ananthapadmanabhan, G Budiono) to screen for eligible studies by applying the inclusion and exclusion criteria described. To maximise inclusivity in the early stage of the systematic review, we included all studies deemed eligible by at least one author. A full-text manuscript of screened articles was then conducted to determine final eligibility for inclusion in the meta-analysis. If disagreements arose, the input of a senior colleague (V Sivapathasingam) was sought until consensus was reached.
Quality assessment
Quality assessment of the included articles was performed using validated, standardized tools. The methodological quality of the included studies was critically appraised using the Joanna Briggs Institute (JBI) Checklist for Prevalence Studies (5) and the quality of the research findings were graded using the Oxford Centre for Evidence-Based Medicine Levels of Evidence (6). The JBI checklist is a validated tool consisting of nine items and scored from 0 to 9, with one point awarded for each item achieved. A high risk of bias was attributed to studies that achieved 5 of less points. The appraisal was performed by two authors (S Ananthapadmanabhan, G Budiono) and if there were discrepancies in identifying the study as high or low risk, the opinion of a senior colleague was consulted. Studies were awarded an evidence grade of 1b for prospective cohort studies or 2b for retrospective cohort studies.
Data extraction
The following data were extracted independently by the authors (S Ananthapadmanabhan, G Budiono) from each study, where reported: total participants, inclusion and exclusion criteria for patient selection, method of diagnosing FCD, incidence of FCD, segment of FCD, whether the operation was primary or revision surgery, proportion of adult and paediatric patients, and the presence of other erosive surgical findings was extracted. The proportion of FCD was calculated from the number of cases with surgical diagnosis of dehiscence of the facial canal divided by the total number of total patients with cholesteatoma who underwent surgery.
Statistical analysis
Statistical analysis was performed using commercially available software STATA version 17.0 (StataCorp LLC, Texas, USA) and Review Manager (Version 5.4, The Cochrane Collaboration, 2020). A P value <0.05 was considered statistically significant and confidence intervals of 95% were used. Forest plots for pooled prevalence were created using the generic inverse variance method with random-effects model. Standard error (SE) to calculate pooled prevalence was calculated using the formula SE = square root of p × (1 − p)/n, where p is the prevalence of FCD within a sample size of n patients. Publication bias was investigated using a funnel plot with Egger’s regression and Begg’s rank test. Leave-one-out (LOO) sensitivity analysis was used to identify studies that disproportionately influenced the pooled prevalence. A subgroup analysis was performed where possible to determine possible sources of heterogeneity in the dataset. Heterogeneity was determined using the τ2, I2, and Q statistic. Meta-regression analysis was performed to determine if moderator variables contributed significantly to data heterogeneity. The sub-groups were divided based on sample size, adults versus paediatric cohort, and primary versus revision surgery. Forest plots of the pooled odds ratio (OR) was calculated using the Mantel-Haenszel test.
Results
Characteristics of included studies
The database search yielded 664 studies from MEDLINE (n=437), PubMed (n=63), Embase (n=164), and Cochrane Library (n=0) and 88 duplicate studies were removed. The PRISMA flow chart for study selection is shown in Figure 1. Twenty-seven articles, including 23 retrospective (3,4,7-27) and 4 prospective (28-31) studies, were suitable for quantitative analysis in the meta-analysis, comprising 5,848 patients with 1,449 instances of FCD. A summary of the characteristics of the included studies is provided in Tables 1,2.
Table 1
| Author, year | Country/region | N | Study type | JBI and Oxford score | Diagnostic method to detect FCD in surgery |
|---|---|---|---|---|---|
| Arias-Marzán, 2019 (7) | Spain | 57 | Retrospective | 5, 2b | Intra-operative microscopic examination |
| Baklacı, 2020 (3) | Turkey | 151 | Retrospective | 6, 2b | Intra-operative microscopic examination and palpation |
| Bayazit, 2002 (8) | Turkey | 49 | Retrospective | 5, 2b | Intra-operative microscopic examination |
| Bizakis, 2006 (9) | Greece | 201 | Retrospective | 4, 2b | Intra-operative examination |
| Bulğurcu, 2017 (27) | Turkey | 245 | Retrospective | 4, 2b | Intra-operative microscopic examination and palpation |
| Choi, 2014 (10) | South Korea | 64 | Retrospective | 4, 2b | Intra-operative microscopic examination and facial nerve monitor |
| Di Martino, 2005 (28) | Germany | 160 | Prospective | 4, 1b | Intra-operative examination |
| Faramarzi, 2017 (11) | Iran | 499 | Retrospective | 6, 2b | Intra-operative microscopic examination and palpation |
| Genc, 2014 (12) | Turkey | 93 | Retrospective | 4, 2b | Intra-operative microscopic examination and palpation |
| Gulotta, Visconti, 2020 (13) | Italy | 527 | Retrospective | 6, 2b | Intra-operative microscopic examination and palpation |
| Gulotta, Pace, 2020 (14) | Italy | 469 | Retrospective | 6, 2b | Intra-operative microscopic examination and palpation |
| Gülüstan, 2014 (15) | Turkey | 334 | Retrospective | 4, 2b | Intra-operative examination |
| Jaswal, 2008 (29) | India | 80 | Prospective | 2, 1b | Intra-operative examination |
| Kalcioglu, 2019 (16) | Turkey | 318 | Retrospective | 6, 2b | Intra-operative microscopic examination |
| Lin, 2004 (17) | Taiwan region | 117 | Retrospective | 7, 2b | Intra-operative microscopic examination |
| Magliulo, 2011 (18) | Italy | 336 | Retrospective | 6, 2b | Intra-operative microscopic examination and palpation |
| Magliulo, 2018 (19) | Italy | 80 | Retrospective | 5, 2b | Intra-operative microscopic examination and palpation |
| Moody, 2007 (30) | USA | 416 | Prospective | 4, 1b | Intra-operative examination |
| Ocak, 2016 (20) | Austria | 50 | Retrospective | 4, 2b | Intra-operative examination |
| Ozbek, 2009 (21) | Turkey | 118 | Retrospective | 5, 2b | Intra-operative microscopic examination and palpation |
| Sahin, 2020, (4) | Turkey | 186 | Retrospective | 6, 2b | Intra-operative microscopic examination and palpation |
| TanrivermiŞ Sayit, 2019 (22) | Turkey | 113 | Retrospective | 4, 2b | Intra-operative examination |
| Selesnick, 2001 (23) | USA | 67 | Retrospective | 4, 2b | Intra-operative examination and palpation |
| Shinnabe, October 2014 (24) | Japan | 310 | Retrospective | 4, 2b | Intra-operative examination and palpation |
| Shinnabe, September 2014 (25) | Japan | 252 | Retrospective | 4, 2b | Intra-operative examination and palpation |
| Trinidade, 2014 (31) | UK | 401 | Prospective | 6, 1b | Intra-operative microscopic examination and palpation |
| Wang, 2006 (26) | Taiwan region | 155 | Retrospective | 7, 2b | Intra-operative microscopic examination |
JBI, joanna briggs institute; FCD, facial canal dehiscence.
Table 2
| Author, year | N | Mean age (years) | Male, n (%) | Paediatric, n (%) | Revision, n (%) | Patient selection | FCD, n (%) |
|---|---|---|---|---|---|---|---|
| Arias-Marzán, 2019 (7) | 57 | 41 | 36 (63.2) | 7 (12.2) | 18 (31.6) | Inclusion: adults and children with primary or revision surgery for cholesteatoma | 17 (29.8) |
| Exclusion: nil | |||||||
| Bias risk: high—does not specify type of cholesteatoma surgery performed | |||||||
| Baklacı, 2020 (3) | 151 | 42 | 85 (56.3) | – | 0 (0) | Inclusion: adults and children with COM with acquired cholesteatoma with primary tympanomastoidectomy | 51 (33.8) |
| Exclusion: history of previous COM surgery, temporal bone trauma, congenital cholesteatoma, other middle ear pathology, lack of preoperative HRCT scans, or congenital inner ear anomalies | |||||||
| Bias risk: high—excludes revision cases and tympanoplasty without mastoidectomy (i.e., small pars tensa cholesteatomas) | |||||||
| Bayazit, 2002 (8) | 49 | – | – | – | – | Inclusion: mixed cohort of 219 adult and children with middle ear surgery for chronic otitis media; 49/219 had cholesteatoma | 9 (18.4) |
| Exclusion: nil | |||||||
| Bias risk: low—operations performed by single surgeon and does not exclude based on revision status or surgery type | |||||||
| Bizakis, 2006 (9) | 201 | 43 | 121 (60.2) | 44 (21.9) | – | Inclusion: adults and children with primary or revision canal wall down mastoidectomy for cholesteatoma | 31 (15.4) |
| Exclusion: canal wall up mastoidectomy | |||||||
| Bias risk: high—only includes CWD mastoid surgery which may overestimate FCD incidence | |||||||
| Bulğurcu, 2017 (27) | 245 | – | – | – | 0 (0) | Inclusion: adults and children with primary tympanomastoidectomy | 49 (20.0) |
| Exclusion: temporal bone fracture, temporal bone neoplasm, tympanoplasty without mastoidectomy, revision surgery, stapes surgery, explorative tympanotomy | |||||||
| Bias risk: high—excludes revision surgery and non-mastoid cholesteatoma surgery | |||||||
| Choi 2014, (10) | 64 | 54 | 29 (45.3) | – | – | Inclusion: mixed cohort of 212 adults and children with chronic otitis media undergoing primary tympanomastoid surgery; 64/212 had cholesteatoma | 41 (64.1) |
| Exclusion: previous otologic surgery, preoperative facial nerve palsy, temporal bone fracture or tumour | |||||||
| Bias risk: high—excludes revision cases and tympanoplasty without mastoidectomy (i.e., small pars tensa cholesteatomas) | |||||||
| Di Martino, 2005 (28) | 160 | – | – | – | 74 (46.3) | Inclusion: comparative study of 357 routine primary or revision middle ear operations, of which 160 had cholesteatoma, versus 300 temporal bone specimens from 150 autopsies | 14 (8.8) |
| Exclusion: history of facial palsy, malformations of facial nerve, temporal bone tumours | |||||||
| Bias risk: low—included different types of cholesteatoma surgery | |||||||
| Faramarzi, 2017 (11) | 499 | 30 | 211 (49.0) | – | 99 (19.8) | Inclusion: 431 adult and children representing 499 ears undergoing primary or revision CWU or CWD tympanomastoidectomy for cholesteatoma | 90 (18.0) |
| Exclusion: inadequate documentation, tympanic surgery only | |||||||
| Bias risk: high—excludes tympanic surgery without mastoidectomy | |||||||
| Genc, 2014 (12) | 93 | – | – | – | 0 | Inclusion: mixed cohort of 154 patients with chronic otitis media, with 93 having cholesteatoma, undergoing primary mastoidectomy | 30 (32.3) |
| Exclusion: previous tympanoplasty or tympanotomy, mastoidectomy, stapes surgery | |||||||
| Bias risk: high—excludes revision surgery and non-mastoidectomy surgery | |||||||
| Gulotta, Visconti, 2020 (13) | 527 | – | 301 (57.1) | 57 (10.8) | – | Inclusion: adult and children with CWU or CWD mastoidectomy for acquired cholesteatoma | 125 (23.7) |
| Exclusion: congenital cholesteatoma, non CWU/CWD surgery | |||||||
| Bias risk: high—excludes non mastoidectomy surgery | |||||||
| Gulotta, Pace, 2020 (14) | 469 | 47 | 267 (56.9) | 0 | 96 (20.5) | Inclusion: adult patients with primary or revision mastoidectomy for cholesteatoma | 121 (25.8) |
| Exclusion: children | |||||||
| Bias risk: high—excludes non mastoidectomy surgery and paediatric patients | |||||||
| Gülüstan, 2014 (15) | 334 | – | 192 (57.5) | 61 (18.3) | 23 (6.9) | Inclusion: adult and children with primary or revision CWU or CWD mastoidectomy for cholesteatoma | 79 (23.7) |
| Exclusion: non CWU/CWD surgery | |||||||
| Bias risk: high—excludes non mastoidectomy surgery | |||||||
| Jaswal, 2008 (29) | 80 | – | – | – | – | Inclusion: mixed cohort of 146 patients with chronic otitis media, with 80 having cholesteatoma, undergoing radical or modified radical mastoidectomy | 18 (22.5) |
| Exclusion: non mastoid surgery | |||||||
| Bias risk: high—excludes non mastoidectomy surgery | |||||||
| Kalcioglu, 2019 (16) | 318 | – | – | – | – | Inclusion: mixed cohort of 372 adult and children undergoing CWU or CWD mastoidectomy for chronic otitis media, with 318 having cholesteatoma | 37 (11.6) |
| Exclusion: non mastoid surgery | |||||||
| Bias risk: high—excludes non mastoidectomy surgery | |||||||
| Lin, 2004 (17) | 117 | – | 49 (41.9) | – | 8 (6.8) | Inclusion: adult and children with primary or revision tympanoplasty with or without mastoidectomy for middle ear cholesteatoma | 39 (33.3) |
| Exclusion: nil | |||||||
| Bias risk: low—included different surgery types | |||||||
| Magliulo, 2011 (18) | 336 | – | 188 (56.0) | 38 (11.3) | 78 (23.2) | Inclusion: adult and children undergoing primary or revision CWU or CWD mastoidectomy for cholesteatoma | 91 (27.1) |
| Exclusion: non mastoid surgery | |||||||
| Bias risk: high—excludes non mastoidectomy surgery | |||||||
| Magliulo, 2018 (19) | 80 | 40 | 48 (60.0) | 0 | 0 | Inclusion: adult patients with attic cholesteatoma who underwent primary microscopic ear surgery and transcanal exclusive endoscopic ear surgery | 16 (20.0) |
| Exclusion: mesotympanum cholesteatoma, wide mastoid involvement, revision surgery | |||||||
| Bias risk: high—excludes non-attic cholesteatoma and revision cases | |||||||
| Moody, 2007 (30) | 416 | – | 237 (57.0) | – | 129 (31.0) | Inclusion: adult and children who underwent tympanoplasty with or without mastoidectomy for cholesteatoma | 78 (18.8) |
| Exclusion: ears previously operated on by same author | |||||||
| Bias risk: high—excludes revision surgery | |||||||
| Ocak, 2016 (20) | 50 | 34 | 31 (62.0) | – | – | Inclusion: mixed cohort of 206 adult and children who underwent tympanoplasty with or without mastoidectomy for various middle ear pathologies; 50/206 had cholesteatoma | 13 (26.0) |
| Exclusion: nil | |||||||
| Bias risk: low—includes different surgery types | |||||||
| Ozbek, 2009 (21) | 118 | – | – | – | – | Inclusion: mixed cohort of 265 adult and children who underwent primary or revision mastoidectomy for middle ear disease, 118/265 had cholesteatoma | 44 (37.3) |
| Exclusion: inadequate documentation, malignant tumours of temporal bone, middle ear surgery without mastoidectomy | |||||||
| Bias risk: high—excludes non mastoidectomy surgery | |||||||
| Sahin, 2020 (4) | 186 | 39 | 118 (63.4) | 19 (10.2) | 61 (32.3) | Inclusion: adult and children with middle ear cholesteatoma with mastoid involvement undergoing primary or revision CWU or CWD mastoidectomy | 68 (36.6) |
| Exclusion: non mastoid surgery | |||||||
| Bias risk: high—excludes non mastoidectomy surgery | |||||||
| TanrivermiŞ Sayit, 2019 (22) | 113 | 36 | 76 (67.3) | – | 0 | Inclusion: adult and children with middle ear cholesteatoma undergoing primary surgery | 62 (54.9) |
| Exclusion: revision surgery | |||||||
| Bias risk: high—excludes revision surgery | |||||||
| Selesnick, 2001 (23) | 67 | – | – | – | – | Inclusion: adult and children who underwent primary or revision cholesteatoma surgery | 22 (32.8) |
| Exclusion: nil | |||||||
| Bias risk: low—includes different surgery types | |||||||
| Shinnabe, October 2014 (24) | 310 | 9.2 (paed) | – | 37 (11.9) | – | Inclusion: adult and children with cholesteatoma who had tympanoplasty | 97 (31.3) |
| 45 (adult) | Exclusion: nil | ||||||
| Bias risk: high—did not specify revision status of patients | |||||||
| Shinnabe, September 2014 (25) | 252 | 42 | – | – | – | Inclusion: adult and children with either pars tensa or pars flaccida cholesteatoma | 85 (33.7) |
| Exclusion: mixed pars tensa and flaccida cholesteatoma | |||||||
| Bias risk: high—excludes two route cholesteatomas | |||||||
| Trinidade, 2014 (31) | 401 | 35.9 | 237 (59.1) | – | – | Inclusion: mastoidectomy surgery for cholesteatoma | 76 (19.0) |
| Exclusion: nil | |||||||
| Bias risk: high—excludes non mastoidectomy surgery | |||||||
| Wang, 2006 (26) | 155* | – | 65 (42.8) | – | 10 (6.5) | Inclusion: primary or revision tympanoplasty with or without mastoidectomy for cholesteatoma | 46 (29.7) |
| Exclusion: nil | |||||||
| Bias risk: low—includes different surgery types |
*, 152 patients representing 155 cases of primary or revision tympanoplasty with or without mastoidectomy for cholesteatoma. COM, chronic otitis media; CWD, canal wall down; CWU, canal wall up; FCD, facial canal dehiscence; HRCT, high-resolution computed tomography; paed, paediatric.
Bias assessment of included studies
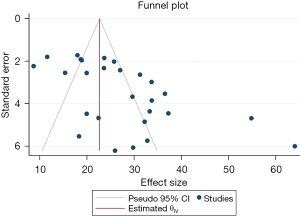
A funnel plot (Figure 2) demonstrated an asymmetrical distribution of effect sizes with most studies plotted outside the 95% confidence interval (CI) limit lines. Egger and Begg’s test for publication bias was Z=3.47, P=0.0005 and Z=2.46, P=0.014 respectively. A small-study effect was evident. Publication bias is not an expected finding as prevalence studies do not report significance levels or compare variables. It is likely that the asymmetric distribution of the funnel plot is related to the heterogeneity amongst included studies. Methodological diversity related to patient selection and surgery performed was likely the main source of bias leading to moderator variables that created inconsistencies between effect sizes across studies. Each study had specific inclusion and exclusion criteria, detailed in Table 2. Examples of confounding factors that could affect FCD incidence include cohort size, proportion of adult and paediatric patients, primary or revision surgery, location and size of cholesteatoma, and type of middle ear surgery performed. In cases where adequate data with respect to these moderator variables was available, a sub-group analysis was performed. Given the overall heterogeneity, presence of moderator variables, and inter-study variability in patient selection, a random-effects model was used in the meta-analysis.
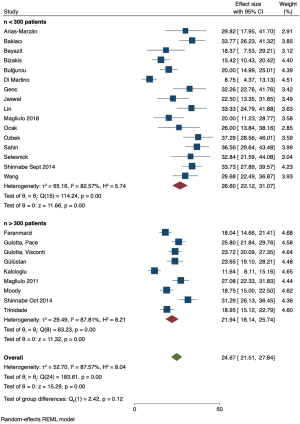
A LOO sensitivity analysis (Figure S1) identified two studies by Choi et al. (10) and TanrivermiŞ Sayit et al. (22) as outliers, which were excluded from the remainder of the analysis. The pooled prevalence changed from 27.21% (95% CI: 22.90–31.51%) to 24.67% (95% CI: 21.51–27.84%) (Figure 3).
Meta-analysis of pooled prevalence and sub-group analysis
The pooled prevalence of FCD was 24.67% (95% CI: 21.51–27.84%) with the representative Forest plot shown in Figure 3B. The Cochran’s Q statistic was 183.61 (df =24, P<0.01) representing significant heterogeneity amongst included studies. The I2 statistic was 87.57% indicating the overall heterogeneity could be accounted for by methodological differences between studies. The proportion of dehiscences that occurred in the tympanic segment of the facial canal was 93.79% (95% CI: 92.06–95.52%), which was consistent amongst the included studies. The tau (τ2) statistic was 2.5, the Cochran’s Q was 15.69 (P=0.27), and the I2 statistic was 24.19%, indicating low heterogeneity.
Sub-group and meta-regression analysis was performed for the moderator variables of primary versus revision surgery, adult versus paediatric patients, and cohort size less than or greater than 300 patients, with representative Forest plots of pooled prevalence and bubble plots of the regression analysis provided (Figures 4-6 and Figures S2-S4 respectively).
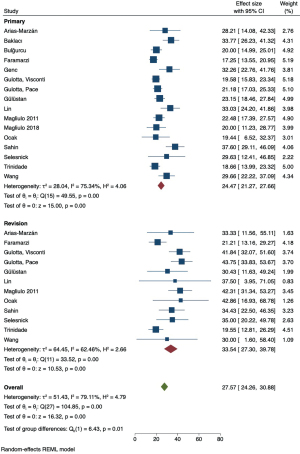
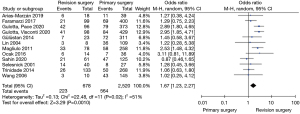
The pooled prevalence of FCD was higher in patients undergoing revision surgery (33.54%, 95% CI: 27.30–39.78%) compared to primary surgery (24.47%, 95% CI: 21.27–27.66%). The test of group difference was significant [Q(1) =6.43, P=0.01]. Meta-regression analysis showed this to be a significant moderator variable contributing to study heterogeneity (Z=−2.57, P=0.01, and R2=21.87%). A meta-analysis of studies that allowed comparison between primary and revision cohorts showed an OR of 1.67 (95% CI: 1.23–2.27) favouring higher FCD incidence in the latter, with a test for overall effect demonstrating significance (Z=3.29, P=0.0010) (Figure 7).
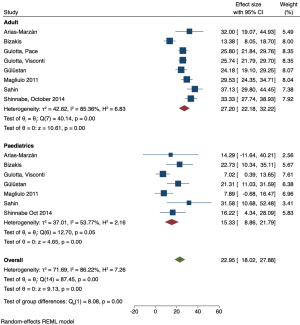
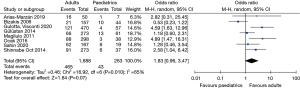
The pooled prevalence of FCD was higher in adult patients (27.20%, 95% CI: 22.18–32.22%) compared to paediatric patients (15.33%, 95% CI: 8.86–21.79%) (Figure 4). A test of group difference was significant [Q(1) =8.08, P<0.01]. Meta-regression analysis showed that age was a significant moderator variable that accounted for some heterogeneity within the analysis (Z=2.78, P=0.005, and R2=42.83%). A meta-analysis of studies that compared reported data for both adult and paediatric patients showed the OR of 1.83 (95% CI: 0.96–3.47) favouring higher FCD incidence in the former, however a test for overall effect was not significant (Z=1.84, P=0.07) and the confidence interval of the summary estimate crossed the line-of-no-effect (Figure 8). A LOO sensitivity analysis (Table S1) identified that this result is likely due to an outlier study that reported a higher dehiscence rate in children (22.7%, n=44) compared to adults (13.4%, n=157) (9). When omitting this study from analysis, we report an OR 2.25 (95% CI: 1.30–3.91) favouring dehiscence in adults which was statistically significant (Z=2.90, P=0.004).
When investigating the effect of sample size, the pooled prevalence of FCD was higher in the smaller studies (26.60%, 95% CI: 22.12–31.07%) compared to larger studies (21.94%, 95% CI: 18.14–25.74%), but a test of group difference was not significant [Q(1) =2.42, P=0.12]. When n=150 and n=200 patients was considered the limit to divide between small and large studies, meta-regression analysis showed no significance in cohort size contributing to the observed heterogeneity.
Twelve studies investigated the association between FCD and lateral semicircular canal fistula (LSCCF) (3,4,11,15,17,18,21,23,25,26,30,31). The pooled prevalence of LSCCF in this cohort was 7.10% (95% CI: 5.57–8.62%) and the pooled prevalence of FCD in patients with LSCCF was 67.84% (95% CI: 57.16–78.51%). The prevalence of LSCCF was likely inflated as most studies had low LSCCF rates that authors did not comment on its association with FCD. Meta-analysis of these 12 studies demonstrated a pooled OR of 6.45 (95% CI: 4.07–10.23) favouring higher incidence of FCD in patients with LSCCF (Figure 9).

Discussion
Summary of main results
This is the first meta-analysis to investigate the pooled prevalence of FCD at the time of cholesteatoma surgery, and we estimate it to be 24.67% (95% CI: 21.51–27.84%). The tympanic segment is consistently demonstrated to be the most common localization of dehiscence with a pooled prevalence of 93.79% (95% CI: 92.06–95.52%). The prevalence of FCD was comparatively higher in adult (27.20%, 95% CI: 22.18–32.22%) versus paediatric (15.33%, 95% CI: 8.86–21.79%) patients and in revision (33.54%, 95% CI: 27.30–39.78%) versus primary (24.47%, 95% CI: 21.27–27.66%) surgery.
FCD in the literature
FCD is a discontinuity in the bony structure of the facial canal, which exposes the facial nerve and may allow herniation into the middle ear cavity. It occurs in cholesteatomatous disease due to two main pathogenic mechanisms (32). Firstly, cholesteatomas are inherently destructive lesions that erode bone by exerting chronic mechanical pressure within the middle ear. Secondly, longstanding inflammation of middle ear mucosa induces osteitis and osteonecrosis of the bony walls of the middle ear and mastoid, with upregulation of osteolytic enzymes, inflammatory mediators, osteoclast-mediated resorptive activity, and adverse bone remodelling processes. FCD increases the risk of preoperative facial nerve palsy, due to inflammatory involvement of the nerve or cholesteatoma invasion into the epineurium, as well as the risk of postoperative palsy, due to accidental surgical injury. Previous studies have estimated the incidence of iatrogenic facial nerve injury to be 0.3–3.6% in primary surgery and 4–10% in revision surgery (33).
Historical studies in cadaveric models of the petrous temporal bone have reported a high incidence of FCD (1,2,34,35). Anatomic and histologic studies by Dietzel et al., Baxter et al., and Moreano et al., estimated a dehiscence rate of 57%, 55%, and 56% respectively (2,34,35). Bilaterality was reported in 76.3% of cases suggesting that the cause of FCD as an anatomic variation in the normal population is likely due to interference in facial canal ossification during foetal development. The authors introduced the concept of microdehiscences, with comparatively higher rates of microdehiscences in adult populations whereas paediatric populations had higher rates of macrodehiscences—this inverse trend is likely due to ongoing ossification in childhood, converting large dehiscences into smaller ones (35). The high incidence in anatomic and histologic studies compared to clinical studies may be due to injuries introduced in the preparation of the specimens. Dehiscences detected in these studies are often microscopic and unnoticeable or of negligible clinical significance to the surgeon, with those less than 1 mm unable to be visualised at the time of surgery. These studies also report a high rate of microdehiscences along the inferior or inferomedial aspect of the tympanic segment, which may be difficult to recognize intraoperatively (2,22,36). Clinical studies investigating FCD in patients undergoing stapes surgery, a cohort used as a surrogate for control or “normal” ears, have reported dehiscence rates of 3.3% by Tange et al. (n=427) (37), 5.2% by Trinidade et al. (n=172) (31), and 11.4% by Li et al. (n=1,465) (38).
The tympanic segment of the facial canal is consistently described as the most common site of dehiscence, with multiple explanations proposed. The bony covering is thinnest in the tympanic segment and most vulnerable to erosion by inflammation and mechanical pressure. Cadaveric models from foetuses and neonates have provided insight into the embryological basis of congenital dehiscences (1,39). Facial canal ossification commences from an apical and canalicular ossification center, proceeding in an anterior-to-posterior direction towards each other (1). Ossification is incomplete at birth and all temporal bones have micro- or macro-dehiscences in the tympanic segment (1), which are considered variations of normal development. Development continues post-partum with fusion of the two ossification centres in the region of the oval window approximately one year after birth and ossification continuing into early childhood replacing macrodehiscences (1,39). It is hypothesized that failure of this fusion is responsible for congenital dehiscences in the tympanic segment, near the oval window niche, and accounts for FCD in individuals without otologic disease (1,39,40). The high rate of tympanic segment dehiscences in this meta-analysis is consistent with current knowledge about the site of origin of cholesteatomas and their expected growth patterns (23,41). Pars tensa cholesteatomas are generally limited to the tympanic cavity, with some extension into the epitympanum, and progress directly around the tympanic facial canal, with posterior mesotympanic variants more likely to involve the posterior tympanic spaces such as the sinus tympani and facial recess (42). Pars flaccida cholesteatomas have more dispersed growth patterns within the tympanum and are more likely to progress towards the aditus ad antrum and the mastoid (43). Finally, because of the tendency for tympanic segment involvement or invasion by cholesteatoma, it is this region where mechanical dissection during surgery is required, introducing risk of creating iatrogenic dehiscences (23).
Multiple studies have cited higher rates of dehiscence in CSOM with cholesteatoma (C-CSOM) compared to CSOM without cholesteatoma (CSOMwoC) (10,12,20,21,29). Genc et al. showed that 88% of CSOM patients with FCD had cholesteatomatous disease, demonstrating a statistically significant association (12). A study by Kalcioglu et al. of 372 tympanomastoidectomy patients showed a similar dehiscence rate with 11.6% (37/318) in the C-CSOM cohort compared to 9.3% (5/54) in the CSOMwoC cohort, with no statistically significant association (P=0.822) (16). However, when analysing adult and paediatric patients separately, the dehiscence rates were significantly higher in the C-CSOM cohort within both sub-groups.
Primary versus revision surgery and FCD
Revision surgery was shown to be significantly associated with higher incidence of FCD compared to primary surgery. This is consistent with previous studies in the literature that have reported higher dehiscence rates at the time of revision cholesteatoma surgery, as shown in Figure 4. This could be explained by (I) surgical trauma due to instrumentation and microdissection of the facial canal during primary surgery, and (II) revision surgery reflecting patients with longer duration and progression of bony erosion in residual or recurrent cholesteatomatous disease. Sahin et al. was the only study in the meta-analysis that had a higher dehiscence rate at primary surgery (37.6%) compared to revision (34.4%), though this was not statistically significant (4). Whilst all other studies reported a higher dehiscence rate in revision surgery, a statistically significant association between FCD and revision surgery was only reported in four studies (13,14,18,20), with the remainder showing no significance (7,11,15,17,23,26,31). This could possibly be explained by the comparatively low patient numbers in the revision cohort, which would require larger magnitude in the difference between dehiscence rates to reach significance, as well as differences amongst authors in the candidates selected for revision surgery, the site and size of the cholesteatoma, and the type of revision surgery performed. Three papers have reported on the association between FCD incidence and disease duration, categorized as greater or less than 5 years, with a significant relationship in each study favouring dehiscence in patients with longer disease burden (13,14,18).
Adult versus paediatrics and FCD
Few studies investigated the association between age and FCD, with the majority reporting higher dehiscence rates in the adult cohort. This observation is likely due to longer disease duration and exposure to chronic inflammation and mechanical pressure in adult patients, and higher likelihood of having previous surgery. This theory is supported by Gulotta et al. (13) who reported a non-significant difference in dehiscence between adults (11.3%, n=106) versus children (6.2%, n=16) in those with disease duration less than 5 years, compared to a significant difference between adults (29.9%, n=364) and children (7.3%, n=41) when disease duration was greater than 5 years. A 2011 study by Magliulo et al. reported a significant OR of 4.96 (95% CI: 1.51–25.97) favouring dehiscence in adults, with this cohort often having more extensive disease (18). They were the only study to look at the effect of primary versus revision surgery in adult and paediatric cohorts, and reported that revision surgery in adult patients had the highest incidence of FCD. Ozbek et al. (21) demonstrated that dehiscence risk increased by 2.88 times in patients over the age of 16 years; however, this included a cohort of mixed middle ear pathologies in which cholesteatoma could not be isolated for analysis. Shinnabe et al. showed a significantly lower incidence of dehiscence in children (16.8%) compared to adults (33.3%) and that FCD rates were influenced by the type of cholesteatoma (24).
FCD and co-existing surgical findings
High resolution CT petrous temporal bones is the standard imaging modality in patients planned for cholesteatoma surgery. Previous studies investigating the radiologic-surgical correlation of diagnosing FCD from preoperative imaging have reported wide variability in sensitivity and specificity (7,18,44-46). This is due to difficulties in evaluating the thin bone of the facial canal, especially in thicker slices where partial volume averaging from adjacent soft tissue may be a confounding factor. Other studies have investigated the role of coexisting surgical findings that may predict FCD—these include the presence of LSCCF or erosion of the scutum (3,12), ossicular chain (4,15,16,27), dural plate of the mastoid tegmen (3,4,11,21,25), or the posterior wall of the external auditory canal (PWEAC) (3,15,29). We report a significant association between LSCCF and FCD in a meta-analysis of 12 studies with an OR 6.45 (95% CI: 4.07–10.23) (3,4,11,15,17,18,21,23,25,26,30,31). Shinnabe et al., demonstrated that the presence of LSCCF was only significant in predicting FCD in pars flaccida cholesteatomas (25). Baklacı (3) and Genc (12) et al. showed an association between FCD and scutum erosion, with 43.2% and 55.6% of patients with scutum erosion respectively having FCD compared to 7.5% and 8.25% of patients without scutum erosion having FCD. This finding is important for pars flaccida cholesteatomas as both studies excluded patients who underwent tympanoplasty without mastoidectomy, resulting in exclusion of small pars tensa cholesteatomas. The relationship between FCD and dehiscence of the dural plate of the mastoid tegmen is uncertain, with some studies reporting significant association (3,4,11,21,25) and others not (26,29). This may be related to the low incidence of dural exposure. The presence of multiple surgical findings raises the likelihood of FCD. A regression analysis by Baklacı et al. (3) showed that LSCCF combined with erosion of the scutum or the PWEAC strongly correlated with FCD with OR 34.3 and 31.6 respectively. Ossicular chain erosion is frequently encountered at the time of cholesteatoma surgery, with the incus being most commonly involved. The presence of a stapes defect, either in isolation or combined with incudal or pan-ossicular erosion, increased the risk of FCD (4,15,16) in three studies, with only Shinnabe et al. (25) showing no association. Hence, these erosive changes, which are often easier to visualize on CT imaging, may be used as cautionary findings to predict encountering FCD intraoperatively.
Study limitations
The limitations of this meta-analysis mainly related to the methodology used in the included studies, especially with respect to patient selection where most studies had a high risk of bias (Table 2). A high degree of heterogeneity in the pooled prevalence of FCD was noted, which was partially explained by the moderator variables investigated in the sub-group analysis. The sensitivity analysis showed two outlier studies, which were excluded from the meta-analysis. Overall, we feel that our estimate of the pooled prevalence should be generalizable despite the observed heterogeneity. Variability in sample sizes introduced a small-study effect, with higher incidence in studies with fewer recruited patients, however this was shown to not be clinically significant on a test of group differences and meta-regression analysis.
In general, the included studies used appropriate selection criterion but some recruited participants with specific indications that can introduce confounding bias into the meta-analysis—for example, the 2018 study by Magliulo et al. (19) exclusively studied attic cholesteatomas, disregarding those arising from the mesotympanum. Most studies did not specify the location or type of the cholesteatoma, whether arising from the pars flaccida or pars tensa, and a comparison between these two entities that demonstrate different growth patterns could not be made. Heterogeneity was apparent with respect to the type of cholesteatoma surgery performed. Whereas some studies exclusively investigated mastoidectomy, others included patients who had tympanoplasty with or without mastoidectomy. It is expected that patients who required mastoidectomy would have had more extensive disease with erosive changes to the facial canal. Studies that excluded patients who had tympanoplasty alone would be omitting small pars tensa cholesteatomas, raising the risk of overestimating FCD incidence. Mastoid segment dehiscences would not be visualised in patients who had tympanoplasty alone, however if a mastoidectomy was not required then it is reasonable to assume that the mastoid facial canal would not have been involved by the cholesteatoma. Data in the literature is not presented in a way to allow subgroup analysis between tympanoplasty with or without mastoidectomy. Size of the dehiscence is not recorded and it is likely that this will be associated with the presence of co-existing destructive surgical findings. FCD may be present as a congenital variant and whether the cholesteatoma is the cause of the dehiscence may be determined by its size, location, and association with other erosive findings.
Whilst all studies stated that dehiscence was diagnosed by intraoperative examination of the facial canal, ten studies did not specifically state the use of the microscope in diagnosis and it is uncertain if these authors used microscopic or endoscopic visualisation. The reported incidence of dehiscence is also dependent on the practice of the operating surgeon, both in terms of detecting and accurately diagnosing it as FCD. There were no standardized criteria amongst different studies and interobserver variability in detecting bony dehiscence is unknown.
In multiple studies, authors presented data in a way that it was difficult to extract data for the sub-group analysis and meta-regression (i.e., they did not separate data by adults versus children or primary versus revision surgery). This was more common in mixed cohorts of cholesteatoma and non-cholesteatomatous ear pathologies. There was inadequate data to compare the effects of primary and revision surgery in children and adults separately.
Overall, this systematic review and meta-analysis highlights that FCD is a common intraoperative finding in cholesteatoma surgery, including up to one-third of revision surgeries. Given, the potential challenges in determining FCD on preoperative imaging, it is important to consider other clinical risk factors and radiographic findings in the patient workup, discussed in our review, to estimate the likelihood of encountering dehiscence. Furthermore, this can preoperative patient counselling about surgical risk and assist with surgical planning, with the option to consider a canal wall down procedure in recurrent cholesteatoma or extensive disease with FCD or other associated cautionary findings.
Conclusions
The pooled prevalence of FCD at the time of cholesteatoma surgery is 24.67%, with the tympanic segment of the facial canal being the most common localisation and adult and revision cases having higher dehiscence rates. Dehiscence of the facial canal may coexist with other destructive findings such as LSCCF and erosion of the ossicular chain erosion, scutum, and PWEAC. The association with revision cases and erosion of the scutum, ossicles, and semicircular canals highlights the importance of thorough preoperative clinical and radiographic assessment to estimate the risk of FCD and prevent injury to the nerve.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://www.theajo.com/article/view/10.21037/ajo-23-1/rc
Data Sharing Statement: Available at https://www.theajo.com/article/view/10.21037/ajo-23-1/dss
Peer Review File: Available at https://www.theajo.com/article/view/10.21037/ajo-23-1/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://www.theajo.com/article/view/10.21037/ajo-23-1/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Approval from the ethics institutional review board was not required for this study as it is a systematic review and meta-analysis of published literature and it does not require collection of patient data.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Spector JG, Ge X. Ossification patterns of the tympanic facial canal in the human fetus and neonate. Laryngoscope 1993;103:1052-65. [Crossref] [PubMed]
- Baxter A. Dehiscence of the Fallopian canal. An anatomical study. J Laryngol Otol 1971;85:587-94. [Crossref] [PubMed]
- Baklacı D, Kuzucu İ, Guler İ, et al. Cautionary High-resolution Computed Tomography Findings for the Presence of Facial Canal Dehiscence in Patients with Cholesteatoma. Cureus 2020;12:e6717. [Crossref] [PubMed]
- Sahin MM, Cayonu M, Dinc ASK, et al. Cautionary Findings for the Presence of Facial Canal Dehiscence During Cholesteatoma Surgery. Ear Nose Throat J 2020;99:327-30. [Crossref] [PubMed]
- Joanna Briggs Institute. Checklist for Prevalence Studies. Available online: https://jbi.global/critical-appraisal-tools (access date: 1st November 2021).
- OCEBM Levels of Evidence Working Group. Oxford Centre for Evidence-Based Medicine: Levels of Evidence (March 2009), Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009 (access date: 1st November 2021).
- Arias-Marzán F, de Lucas-Carmona G, Pacheco Coronel ER, et al. Facial canal dehiscence in patients with cholesteatoma: concordance between intraoperative inspection, computed tomography and neurophysiological findings. Eur Arch Otorhinolaryngol 2019;276:1915-20. [Crossref] [PubMed]
- Bayazit YA, Ozer E, Kanlikama M. Gross dehiscence of the bone covering the facial nerve in the light of otological surgery. J Laryngol Otol 2002;116:800-3. [Crossref] [PubMed]
- Bizakis JG, Chimona TS, Hajiioannou JK, et al. Canal wall down mastoidectomy for cholesteatoma: experience at the University of Crete. J Otolaryngol 2006;35:48-52. [Crossref] [PubMed]
- Choi SA, Kang HM, Byun JY, et al. Analysis of differences in facial nerve dehiscence and ossicular injury in chronic otitis media and cholesteatoma. Acta Otolaryngol 2014;134:455-61. [Crossref] [PubMed]
- Faramarzi M, Roosta S. Incidence of Facial Nerve Canal Dehiscence in Primary and Revision Cholesteatoma Surgery. Indian J Otolaryngol Head Neck Surg 2017;69:300-6. [Crossref] [PubMed]
- Genc S, Genc MG, Arslan IB, et al. Coexistence of scutum defect and facial canal dehiscence. Eur Arch Otorhinolaryngol 2014;271:701-5. [Crossref] [PubMed]
- Gulotta G, Visconti IC, Pace A, et al. Facial nerve dehiscence and cholesteatoma: Pediatrics vs adults. Int J Pediatr Otorhinolaryngol 2020;138:110260. [Crossref] [PubMed]
- Gulotta G, Pace A, Iannella G, et al. Facial Nerve Dehiscence and Cholesteatoma: A Comparison between Decades. J Int Adv Otol 2020;16:367-72. [Crossref] [PubMed]
- Gülüstan F, Aslan H, Songu M, et al. Relationships between facial canal dehiscence and other intraoperative findings in chronic otitis media with cholesteatoma. Am J Otolaryngol 2014;35:791-5. [Crossref] [PubMed]
- Kalcioglu MT, Kilic O, Tuysuz O, et al. Facial canal dehiscence rate: a retrospective analysis of 372 chronic otitis media cases. Eur Arch Otorhinolaryngol 2019;276:79-83. [Crossref] [PubMed]
- Lin JC, Ho KY, Kuo WR, et al. Incidence of dehiscence of the facial nerve at surgery for middle ear cholesteatoma. Otolaryngol Head Neck Surg 2004;131:452-6. [Crossref] [PubMed]
- Magliulo G, Colicchio MG, Appiani MC. Facial nerve dehiscence and cholesteatoma. Ann Otol Rhinol Laryngol 2011;120:261-7. [Crossref] [PubMed]
- Magliulo G, Iannella G. Endoscopic versus microscopic approach in attic cholesteatoma surgery. Am J Otolaryngol 2018;39:25-30. [Crossref] [PubMed]
- Ocak E, Beton S, Mulazimoglu S, et al. Does Dehiscence of the Facial Nerve Canal Affect Tympanoplasty Results? J Craniofac Surg 2016;27:e374-6. [Crossref] [PubMed]
- Ozbek C, Tuna E, Ciftci O, et al. Incidence of fallopian canal dehiscence at surgery for chronic otitis media. Eur Arch Otorhinolaryngol 2009;266:357-62. [Crossref] [PubMed]
- Tanrivermi Ş. Association between facial nerve second genu angle and facial canal dehiscence in patients with cholesteatoma: evaluation with temporal multidetector computed tomography and surgical findings. Braz J Otorhinolaryngol 2019;85:365-70. [Crossref] [PubMed]
- Selesnick SH, Lynn-Macrae AG. The incidence of facial nerve dehiscence at surgery for cholesteatoma. Otol Neurotol 2001;22:129-32. [Crossref] [PubMed]
- Shinnabe A, Yamamoto H, Hara M, et al. Fallopian canal dehiscence at pediatric cholesteatoma surgery. Eur Arch Otorhinolaryngol 2014;271:2927-30. [Crossref] [PubMed]
- Shinnabe A, Yamamoto H, Hara M, et al. Differences in clinical characteristics of fallopian canal dehiscence associated with pars flaccida and pars tensa cholesteatomas. Eur Arch Otorhinolaryngol 2014;271:2171-5. [Crossref] [PubMed]
- Wang HM, Lin JC, Lee KW, et al. Analysis of mastoid findings at surgery to treat middle ear cholesteatoma. Arch Otolaryngol Head Neck Surg 2006;132:1307-10. [Crossref] [PubMed]
- Bulğurcu S, Arslan İB, Dikilitaş B, et al. Relation between Ossicular Erosion and Destruction of Facial and Lateral Semicircular Canals in Chronic Otitis Media. Int Arch Otorhinolaryngol 2017;21:239-42. [Crossref] [PubMed]
- Di Martino E, Sellhaus B, Haensel J, et al. Fallopian canal dehiscences: a survey of clinical and anatomical findings. Eur Arch Otorhinolaryngol 2005;262:120-6. [Crossref] [PubMed]
- Jaswal A, Jana AK, Sikder B, et al. Fallopian canal dehiscence: can it be pridicted. Indian J Otolaryngol Head Neck Surg 2008;60:11-5. [Crossref] [PubMed]
- Moody MW, Lambert PR. Incidence of dehiscence of the facial nerve in 416 cases of cholesteatoma. Otol Neurotol 2007;28:400-4. [Crossref] [PubMed]
- Trinidade A, Yung MW. The intra-operative incidence of Fallopian canal dehiscence during surgery for cholesteatoma: a prospective case-control study and review of the literature. Clin Otolaryngol 2014;39:138-44. [Crossref] [PubMed]
- Hamed MA, Nakata S, Sayed RH, et al. Pathogenesis and Bone Resorption in Acquired Cholesteatoma: Current Knowledge and Future Prospectives. Clin Exp Otorhinolaryngol 2016;9:298-308. [Crossref] [PubMed]
- Wiet RJ. Iatrogenic facial paralysis. Otolaryngol Clin North Am 1982;15:773-80. [Crossref] [PubMed]
- Dietzel K. On dehiscence of the facial nerve canal. Z Laryngol Rhinol Otol 1961;40:366-79. [PubMed]
- Moreano EH, Paparella MM, Zelterman D, et al. Prevalence of facial canal dehiscence and of persistent stapedial artery in the human middle ear: a report of 1000 temporal bones. Laryngoscope 1994;104:309-20. [Crossref] [PubMed]
- Takahashi H, Sando I. Facial canal dehiscence: histologic study and computer reconstruction. Ann Otol Rhinol Laryngol 1992;101:925-30. [Crossref] [PubMed]
- Tange RA, de Bruijn AJ. Dehiscences of the horizontal segment of the facial canal in otosclerosis. ORL J Otorhinolaryngol Relat Spec 1997;59:277-9. [Crossref] [PubMed]
- Li D, Cao Y. Facial canal dehiscence: a report of 1,465 stapes operations. Ann Otol Rhinol Laryngol 1996;105:467-71. [Crossref] [PubMed]
- Weiglein AH. Postnatal development of the facial canal. An investigation based on cadaver dissections and computed tomography. Surg Radiol Anat 1996;18:115-23. [Crossref] [PubMed]
- Perez B, Campos ME, Rivero J, et al. Incidence of dehiscences in the fallopian canal. Int J Pediatr Otorhinolaryngol 1997;40:51-60. [Crossref] [PubMed]
- Jackler RK. The surgical anatomy of cholesteatoma. Otolaryngol Clin North Am 1989;22:883-96. [Crossref] [PubMed]
- Kole SA, Nieland PPF, Søvsø M, et al. Incidence of middle-ear cholesteatoma with analysis of its locations, extensions, and complications from 1993 to 2009. In: Takahashi H. editor. Cholesteatoma and Ear Surgery - An Update: Proceedings of the 9th International Conference on Cholesteatoma and ear surgery. Kugler Publications, 2013:297-301.
- Pusalkar AG. Cholesteatoma and Its Management. Indian J Otolaryngol Head Neck Surg 2015;67:201-4. [Crossref] [PubMed]
- Yu Z, Wang Z, Yang B, et al. The value of preoperative CT scan of tympanic facial nerve canal in tympanomastoid surgery. Acta Otolaryngol 2011;131:774-8. [Crossref] [PubMed]
- Gül A, Akdağ M, Kiniş V, et al. Radiologic and surgical findings in chronic suppurative otitis media. J Craniofac Surg 2014;25:2027-9. [Crossref] [PubMed]
- Kanotra S, Gupta R, Gupta N, et al. Correlation of high-resolution computed tomography temporal bone findings with intra-operative findings in patients with cholesteatoma. Indian J Otol 2015;21:280-5. [Crossref]
Cite this article as: Ananthapadmanabhan S, Budiono G, Jabbour J, Ayeni FE, King G, Suruliraj A, Sivapathasingam V. Facial canal dehiscence in cholesteatoma and co-existing surgical findings: a systematic review and meta-analysis. Aust J Otolaryngol 2023;6:13.


