Clinical decision making for anterior and posterior lingual abscess: a systematic review
Introduction
Lingual abscess is a rare clinical entity defined as an infectious process within the tongue parenchyma (1,2). The tongue has many protective mechanisms against trauma and foreign pathogen such as keratinized epithelium, rich vascular supply plus lymphatic drainage, thick musculature, and the antimicrobial properties of saliva. A lingual abscess may occur in the anterior two thirds or posterior aspect of the tongue. Posteriorly located abscesses may pose more clinical uncertainty in diagnosis compared to those located anteriorly. The formation of a lingual abscess is likely due to the dysfunction and/or disruption of the inherit protective features and mechanisms associated (1). An abscess located anteriorly may be associated with local trauma, odontogenic infections and penetration of foreign bodies as opposed to a posterior location with factors such as pharyngitis/tonsilitis and infected thyroglossal cysts (1,3,4).
Lingual abscesses present an increased risk of airway compromise and therefore should be assessed and treated promptly to reduce morbidity and mortality. In the pre-antibiotic era, lingual abscesses had a mortality rate of 3%, now in the modern era with antibiotic treatment and advanced imaging techniques an improvement in overall mortality rate is difficult to determine due to limited case reports (5). The vast majority of the literature are case reports with little consensus on aetiology, clinical characteristics, and management. This systematic review will critically analyse the clinical presentation, investigations, and management, with a particular focus on the differences between an anterior and posterior lingual abscess.
Methods
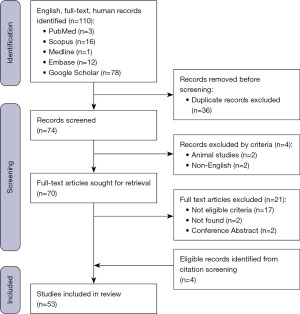
A systematic review was conducted on the 2nd of January 2023 using the terms “lingual abscess”, and “tongue abscess” across the databases PubMed, SCOPUS, Medline, Embase and Google Scholar. The protocol of this systematic review was published online at the International Prospective Register of Systematic Reviews (PROSPERO) under registration number CRD42023396816. The inclusion criteria were confirmed cases of a lingual abscess published in case reports or case series. All articles within the published literature between 1970 and 2022 were eligible for inclusion. The exclusion criteria were articles not published in English, no reported management, and review articles. Search results were reviewed independently by both reviewers based on title and abstract, with subsequent full-text screening of potentially eligible articles to determine inclusion. The expertise of senior surgical colleagues was available if uncertainty arose. We present this article in accordance with the PRISMA reporting checklist (available at https://www.theajo.com/article/view/10.21037/ajo-23-13/rc) (6). The Joanna Briggs Institute checklist, standardized tool to assess risk of bias for case reports and case series was used to assess for risk of bias and is provided in Figure S1 (7).
For included studies, data extraction was conducted independently by one author and crosschecked by another. Data extracted from eligible studies included patient characteristics, clinical presentation, radiological evaluation, abscess location, predisposing risk factors, management and perioperative morbidity and mortality. In addition, antibiotics treatment used, and any pathogen confirmed from investigative cultures.
Statistical analyses were performed using IBM SPSS Statistics version 28.0 (IBM, New York, NY, USA). Continuous variables were expressed as mean, and standard deviation. The differences between proportions for anterior and posterior abscess groups were analysed using Chi-square test and a student t-test for categorical and continuous variables, respectively. The level of statistical significance was set at P value of 0.05. Ethics approval was not required for this study. Because of the retrospective nature of the research, the requirement for informed consent was waived.
Results
Study selection
A total of 53 studies were included in the present review (Figure 1). Forty-two articles were case reports and 11 were case series, reporting on a total of 73 cases of lingual abscess. Nineteen studies originated from Asia, 16 from North America, 15 from Europe and the remaining 3 from Africa (Table 1).
Table 1
| Study | Year |
|---|---|
| Jain (8) | 1970 |
| Palestini (9) | 1981 |
| Eames (10) | 1983 |
| Legget (11) | 1987 |
| Roberge (12) | 1989 |
| Sands (4) | 1993 |
| Renehan (13) | 1993 |
| Hehar (14) | 1996 |
| Jungell (15) | 1996 |
| Muñoz (16) | 1998 |
| Olsen (17) | 2001 |
| Brook (18) | 2002 |
| Eviatar (19) | 2004 |
| Antoniades (20) | 2004 |
| Balatsouras (21) | 2004 |
| de Waal (22) | 2004 |
| Kim (23) | 2006 |
| Kiroglu (24) | 2006 |
| Boon (3) | 2009 |
| Nariai (25) | 2010 |
| Tajudeen (26) | 2011 |
| Vellin (27) | 2011 |
| Byahatti (28) | 2011 |
| Veloo (29) | 2011 |
| Harrington (30) | 2012 |
| Pallagatti (31) | 2012 |
| Barrueco (32) | 2012 |
| Kikidis (33) | 2012 |
| Solomon (34) | 2012 |
| Kulkarni (35) | 2013 |
| Burnham (36) | 2013 |
| Varghese (37) | 2013 |
| Kettaneh (38) | 2014 |
| Coughlin (39) | 2014 |
| Ozgur (40) | 2015 |
| Lefler (41) | 2016 |
| Pandey (42) | 2016 |
| Kuge (43) | 2017 |
| Bekele (44) | 2017 |
| Al-Anee (45) | 2018 |
| Gama (46) | 2018 |
| Potigailo (47) | 2018 |
| Srivanitchapoom (1) | 2018 |
| Tewari (48) | 2018 |
| Schweigert (5) | 2020 |
| Akin (49) | 2020 |
| Araidy (50) | 2020 |
| Mesolella (51) | 2021 |
| Wong (52) | 2021 |
| Bülbül (53) | 2021 |
| Carotenuto (2) | 2022 |
| Mesfin (54) | 2022 |
| Little (55) | 2022 |
Patient characteristics
Of the 73 patients diagnosed, the mean age was 42 (±19) years with a male predominance (n=46, 63.0%). Poor oral hygiene was reported in 23 patients (31.5%) with other noted risk factors including diabetes mellitus (n=9, 12.3%) and immunocompromise (n=4, 5.5%). The location of the abscess was reported in 45 (61.6%) anterior, 26 posterior (35.6%) and 2 (2.8%) involving the entirety the tongue (Table 2).
Table 2
| Clinical variables | Values |
|---|---|
| Demographic data | |
| Age (years), mean (SD) | 41.8 (18.8) |
| Sex | |
| Male | 46 (63.0%) |
| Female | 27 (37.0%) |
| Abscess location | |
| Anterior | 45 (61.6%) |
| Posterior | 26 (35.6%) |
| Anterior + posterior | 2 (2.8%) |
| Symptoms and signs | |
| Tongue swelling | 68 (93.2%) |
| Odynophagia | 38 (52.1%) |
| Dysphagia | 40 (54.8%) |
| Dyspnoea | 9 (12.3%) |
| Dysphonia | 16 (21.9%) |
| Sialorrhoea | 7 (9.6%) |
| Otalgia | 8 (11.0%) |
| Localised tongue pain | 31 (42.5%) |
| Airway obstruction | 17 (23.3%) |
| Surrounding structure involvement | |
| Total | 16 (21.9%) |
| Sublingual | 6 |
| Pharynx | 4 |
| Epiglottis | 5 |
| Submental | 1 |
| Predisposing factors | |
| Not specified | 40 (54.8%) |
| Trauma | 12 (16.4%) |
| Dentition/tongue bite | 6 |
| Medical/dental procedure | 4 |
| Other | 2 |
| Foreign body | 9 (12.3%) |
| Fish bone | 6 |
| Other | 3 |
| Odontogenic infections/caries | 7 (9.6%) |
| Recent pharyngitis/tonsilitis | 4 (5.5%) |
| Oro-motor dysfunction (neuromuscular disease) | 1 (1.4%) |
| Reported risk factors | |
| Poor oral hygiene | 23 (31.5%) |
| Immunocompromised | 4 (5.5%) |
| Diabetes mellitus | 9 (12.3%) |
| Imaging | |
| Computed tomography | 39 (53.4%) |
| X-ray | 3 (4.1%) |
| Ultrasound | 8 (11.0%) |
| Magnetic resonance imaging | 7 (9.6%) |
| Nil | 21 (28.8%) |
| Flexible nasopharyngoscopy | 2 (2.7%) |
| Management | |
| Aspiration | 33 (45.8%) |
| Incision and drainage | 45 (62.5%) |
| Not drained | 1 (1.4%) |
| Unknown | 1 (1.4%) |
| Anesthesia | |
| Local | 40 (56.3%) |
| General | 30 (42.3%) |
| None | 1 (1.4%) |
| Airway management | |
| Endotracheal tube | 13 (17.8%) |
| Nasotracheal tube | 1 (1.4%) |
| Tracheostomy | 8 (11.0%) |
| None | 44 (60.3%) |
| Unknown | 7 (9.6%) |
| Morbidity | 4 (5.5%) |
| Recurrence | 3 |
| Sepsis | 1 |
| Mortality | 1 (1.4%) |
SD, standard deviation.
Clinical presentation
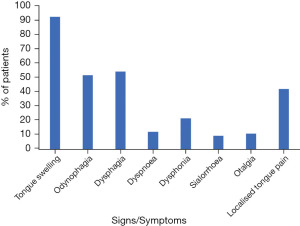
The most prevalent reported symptoms included tongue swelling (n=68, 93.2%), dysphagia (n=40, 54.8%), odynophagia (n=38, 52.1%) and localised tongue pain (n=31, 42.5%). Less common symptoms included dysphonia (n=16, 21.9%), dyspnoea (n=9, 12.3%), otalgia (n=8, 11.0%) and sialorrhoea (n=7, 9.6%) (Figure 2). A posterior lingual abscess was significantly more likely to report symptoms of otalgia (25.9% vs. 2.2%, P=0.002) and sialorrhoea (18.5% vs. 4.3%, P=0.047, Table 3) than an anterior based abscess. Airway obstruction of any degree was reported in 17 (23.3%) patients with no significant difference between anterior and posterior lingual abscess (19.6% vs. 29.6%, P=0.326, Table 3).
Table 3
| Clinical variables | Anterior abscess (n=46) | Posterior abscess (n=27) | P value |
|---|---|---|---|
| Demographic data | |||
| Age (years), mean (SD) | 40.5 (19.8) | 44.1 (17.1) | 0.448 |
| Male | 28 (60.9%) | 18 (66.7%) | 0.620 |
| Female | 18 (39.1%) | 9 (33.3%) | |
| Symptoms and signs | |||
| Tongue swelling | 43 (93.5%) | 25 (92.6%) | 0.885 |
| Odynophagia | 21 (45.7%) | 17 (63.0%) | 0.153 |
| Dysphagia | 22 (47.8%) | 18 (66.7%) | 0.118 |
| Dyspnoea | 6 (13.0%) | 3 (11.1%) | 0.808 |
| Dysphonia | 10 (21.7%) | 6 (22.2%) | 0.962 |
| Sialorrhoea | 2 (4.3%) | 5 (18.5%) | 0.047* |
| Otalgia | 1 (2.2%) | 7 (25.9%) | 0.002* |
| Localised tongue pain | 23 (50.0%) | 8 (29.6%) | 0.089 |
| Predisposing factors | |||
| Trauma | 9 (19.6%) | 3 (11.1%) | 0.341 |
| Foreign body | 6 (13.0%) | 3 (11.1%) | 0.808 |
| Odontogenic infections/caries | 5 (10.9%) | 2 (7.4%) | 0.628 |
| Recent pharyngitis/tonsilitis | 1 (2.2%) | 3 (11.1%) | 0.105 |
| Airway obstruction | 9 (19.6%) | 8 (29.6%) | 0.326 |
| Surrounding structure involvement | |||
| Sublingual | 5 (10.9%) | 1 (3.7%) | 0.282 |
| Pharynx | 1 (2.2%) | 3 (11.1%) | 0.105 |
| Epiglottis | 0 (0.0%) | 5 (18.5%) | 0.002* |
| Submental | 0 (0.0%) | 1 (3.7%) | 0.189 |
| Imaging | |||
| Computed tomography | 16 (34.8%) | 23 (85.2%) | <0.001* |
| Plain radiography | 3 (6.5%) | 0 (0.0%) | 0.175 |
| Ultrasound | 8 (17.4%) | 0 (0.0%) | 0.022* |
| Magnetic resonance imaging | 4 (8.7%) | 3 (11.1%) | 0.735 |
| Nil | 19 (41.3%) | 2 (7.4%) | 0.002* |
| Flexible nasopharyngoscopy | 0 (0.0%) | 2 (7.4%) | 0.061 |
| Management | |||
| Aspiration | 18 (39.1%) | 15 (55.6%) | 0.173 |
| Incision and drainage | 31 (67.4%) | 14 (51.9%) | 0.187 |
| Pathogen | |||
| Gram positive | 11 (23.9%) | 10 (37.0%) | 0.232 |
| Gram negative | 5 (10.9%) | 2 (7.4%) | 0.628 |
| Mixed growth | 11 (23.9%) | 4 (14.8%) | 0.353 |
| Antibiotics | |||
| Penicillin | 4 (8.7%) | 1 (3.7%) | 0.415 |
| Penicillin + gram-negative cover† | 13 (28.3%) | 3 (11.1%) | 0.087 |
| Broad spectrum‡ | 28 (60.9%) | 21 (77.8%) | 0.138 |
| Morbidity | 3 (6.5%) | 1 (3.7%) | 0.377 |
| Mortality | 0 (0.0%) | 1 (3.7%) | 0.189 |
†, classified if stated as such or prescribed cephalosporin, quinolones and antimetabolites in varying combinations, e.g., ceftriaxone + cephalexin, amoxicillin-clavulanic acid + ceftriaxone; ‡, classified if prescribed aminopenicillins with beta-lactamase inhibitor +/− gentamicin, e.g., ampicillin-sulbactam, ampicillin-cloxacillin, penicillin + gentamicin; *, P<0.05. SD, standard deviation.
There were no known predisposing factors to lingual abscess formation reported in 37 (50.7%) cases. Known predisposing factors included trauma (n=12, 16.4%), foreign body (n=9, 12.3%), odontogenic infections/caries (n=7, 9.6%), recent pharyngitis and/or tonsilitis (n=4, 5.5%) and one reported case of oro-motor dysfunction attributed to underlying bulbar amyotrophic lateral sclerosis (ALS).
The most common imaging modality utilised included computed tomography (CT) (n=39, 53.4%), followed by ultrasound (n=8, 11.0%), magnetic resonance imaging (n=7, 9.6%), plain radiography (n=3, 4.1%). On presentation two patients underwent flexible nasopharyngoscopy as part of the investigative work-up. Twenty-one patients (28.8%) did not undergo any form of imaging during investigation. A posterior lingual abscess was significantly more likely to have CT compared to the anterior cohort (85.2% vs. 34.8%, P<0.001, Table 3). Comparatively, ultrasound was significantly more likely to be used with an anterior based abscess than posterior (17.4% vs. 0.0%, P=0.022, Table 3). Furthermore, an anterior based abscess was significantly more likely to not have imaging compared to a posterior abscess (41.3% vs. 7.4%, P=0.002). Oedema and/or cellulitis of pharynx, epiglottis, sublingual, and submental areas were reported in 16 (21.9%) cases. A posterior lingual abscess was significantly more likely to have involvement of the epiglottis than an anterior based abscess (18.5% vs. 0.0%, P=0.002).
Management
All but two cases underwent aspiration (45.8%) or incision and drainage (62.5%). There was no significant difference between these management options in an anterior versus posterior location (P>0.05, Table 3). A tracheostomy was performed on 8 (11.0%) patients in this cohort. In the cases that reported bacterial cultures, gram-positive organisms were most common (48.8%), followed by mixed growths (34.9%) and gram negative (16.3%). There was no significant difference in isolated pathogens in these categories when comparing an anterior and posterior lingual abscess (P>0.05, Table 3). The most common individual isolated pathogens reported included Fusobacterium nucleatum (7.0%) and Streptococcus viridans (7.0%) (Table 4). The three most common antibiotics utilised included amoxicillin-clavulanic acid (21.9%), ceftriaxone (21.9%) and metronidazole (21.9%) (Table 5). There was no significant difference in antimicrobial prescribing practices between an anterior versus posterior lingual abscess (P>0.05, Table 3).
Table 4
| Pathogen | Values |
|---|---|
| Unknown | 16 (22.5%) |
| No growth | 12 (16.9%) |
| Fusobacterium nucleatum | 5 (7.0%) |
| Streptococcus viridans | 5 (7.0%) |
| Mixed oral flora | 4 (5.6%) |
| Gram positive cocci | 4 (5.6%) |
| Streptococci | 4 (5.6%) |
| Bacteroides spp | 3 (4.2%) |
| Prevotella | 3 (4.2%) |
| Streptococcus anginosus | 3 (4.2%) |
| Anaerobes | 2 (2.8%) |
| Gram negative anaerobes | 2 (2.8%) |
| Peptostreptococcus | 2 (2.8%) |
| Staphylococci | 2 (2.8%) |
| Staphylococcus aureus | 2 (2.8%) |
| Streptococcus faecalis | 2 (2.8%) |
| Streptococcus haemolyticus | 2 (2.8%) |
| Acinetobacter lwoffii | 1 (1.4%) |
| Bacteroides ureolyticus | 1 (1.4%) |
| Beta-haemolytic non-group A, B, D | 1 (1.4%) |
| Candida albicans | 1 (1.4%) |
| Enterococci | 1 (1.4%) |
| Gram positive anaerobes | 1 (1.4%) |
| Group B Streptococcus | 1 (1.4%) |
| Group D beta-haemolytic streptococci | 1 (1.4%) |
| Haemophilus aphrophilus | 1 (1.4%) |
| Haemophilus parainfluenzae | 1 (1.4%) |
| Klebsiella ozaenae | 1 (1.4%) |
| Neisseria | 1 (1.4%) |
| Porphyromonas | 1 (1.4%) |
| Prevotella melaninogenica | 1 (1.4%) |
| Staphylococcus epidermidis | 1 (1.4%) |
| Streptococcus agalactiae | 1 (1.4%) |
| Streptococcus intermedius | 1 (1.4%) |
| Streptococcus pyogenes | 1 (1.4%) |
Table 5
| Antibiotic | Values |
|---|---|
| Unknown | 17 (23.3%) |
| Amoxicillin-clavulanic acid | 16 (21.9%) |
| Ceftriaxone | 16 (21.9%) |
| Metronidazole | 16 (21.9%) |
| Clindamycin | 14 (19.2%) |
| Penicillin | 7 (9.6%) |
| Cefazolin | 3 (4.1%) |
| Amikacin | 3 (4.1%) |
| Phenoxymethylpenicillin | 2 (2.7%) |
| Cefuroxime | 2 (2.7%) |
| Amoxicillin | 2 (2.7%) |
| Gentamicin | 2 (2.7%) |
| Vancomycin | 2 (2.7%) |
| Ampicillin-sulbactam | 2 (2.7%) |
| Piperacillin-tazobactam | 2 (2.7%) |
| Cephalexin | 1 (1.4%) |
| Ceforanide | 1 (1.4%) |
| Trimethoprim-sulfamethoxazole | 1 (1.4%) |
| Ticarcillin-clavulanic acid | 1 (1.4%) |
| Cloxacillin | 1 (1.4%) |
| Linezolid | 1 (1.4%) |
| Piperacillin | 1 (1.4%) |
| Ampicillin-cloxacillin | 1 (1.4%) |
| Teicoplanin | 1 (1.4%) |
Discussion
We identified that a posterior lingual abscess significantly differs in its clinical presentation and use of imaging with increased risk of surrounding tissue involvement compared to an anterior based lingual abscess. The literature highlights that lingual abscess formation is more common in males (1,8,19) with a strong male predominance seen in this cohort (63.0%) and an average age of 42 (±19) years. Our review currently highlights no significant difference in male or female predominance when it comes to an anterior versus posterior lingual abscess (P>0.05, Table 3). The literature reports that an anterior abscess is often more associated with trauma, odontogenic infections and foreign bodies as opposed to posterior associated with surround tissue infection (tonsils, pharynx) (1,3,4). However, the formation of a spontaneous abscess of unknown aetiology has been reported in the majority of reported cases, with no predisposing factor found in 37 cases (50.7%) within the current review (19,30). In one case Lefler reported an abscess formation was attributed to recent oral antibiotic injections, while Tajudeen and colleagues reported a case of anterior abscess attributed to a retained suture from a lingual procedure 2 years prior (26,41). Our review did not find any of the above factors more likely to predispose a patient to an anterior compared to a posterior lingual abscess (P>0.05, Table 3). Poor oral hygiene, immunocompromised states and chronic disease such as diabetes mellitus may also play a role for predisposition to abscess formation (1,20,30).
Patients with a reported lingual abscess may present with one or combination of signs and symptoms (Table 2). An anterior abscess typically presents with odynophagia, dysphagia, localised pain, dysphonia and tongue swelling (19-21,51,52). Whereas, a posterior abscess clinically may be difficult to diagnose in comparison and present with symptoms of otalgia, sialorrhoea and painful tongue protrusion (1,21,27). The posterior tongue, internal tympanic membrane and portions of the middle ear are supplied by the glossopharyngeal nerve and the suggested mechanism of referred pain from the posterior lingual abscess to the ear (3). We confirmed these findings showing that a posterior lingual abscess was significantly more likely to report otalgia and sialorrhoea compared to an anterior abscess (P<0.05, Table 3). Given the less overt clinical signs and the potential sequelae, we recommend any patients presenting with tongue swelling and/or localised pain in combination with either one or both symptoms of otalgia and sialorrhoea have posterior lingual abscess on the list of differential diagnoses. Harrington and colleagues emphasized that there was an increased potential for airway obstruction in cases of posterior lingual abscess, while Srivanitchapoom reported impending airway obstruction upon presentation of an anteriorly located abscess (1,30). We reported no significant difference in airway obstruction for an anterior versus posterior abscess. However, the potential spread of the infection to surrounding soft tissue and structures may increase the risk of airway obstruction. The involvement of the epiglottis was only seen in cases of posterior lingual abscess in this current review. This is thought to be due to the lymphatic drainage of the posterior tongue to deep cervical lymph nodes as opposed to the anterior aspect drainage to submental and submandibular lymph nodes. With confluent collection from the posterior tongue to the pre-epiglottic region, deep cervical space infection can often have rapid onset and life-threating complications including airway compromise (56). Timely diagnosis is needed in cases of posterior lingual abscess as the potential for surrounding tissues involvement is greater compared to an anterior presentation. Surrounding structure involvement may be underestimated given the proportion of patients (28.8%) did not receive some form of radiographical imaging.
A comprehensive clinical history and physical exam, including lingual palpation can aid in the diagnosis, particularly in cases of an anterior located abscess without the need for imaging. Several studies reported patients undergoing more than one imaging modality during investigations (23,27,35,43,51). Our review showed it was significantly more likely to identify an anterior tongue abscess based on clinical examination and/or ultrasound compared to a posterior abscess (41.3% vs. 7.4%, P=0.002, Table 3). In a posterior based abscess, the ability to confidently diagnose and exclude deep seated infections or malignancy can be challenging (3,4,19,21,27). We found that posterior abscess patients were significantly more likely to undergo CT compared to an anterior abscess (34.8% vs. 85.2%, P<0.001, Table 3). This is attributed to the difficulty in visualization of posterior tongue structures on clinical exam, whereby only 2 cases reported the use of nasoendoscopy with no airway obstruction reported (21,51). In these cases, we recommend a clinical algorithm to aid in the diagnosis of a lingual abscess and if radiographic imaging is warranted (Figure 3). It outlines key differences that may be associated with a posterior lingual abscess to minimise the risk of a missed diagnosis. In cases with tongue swelling and pain without evidence of collection we advocate for airway monitoring due to high risk of impending compromise.
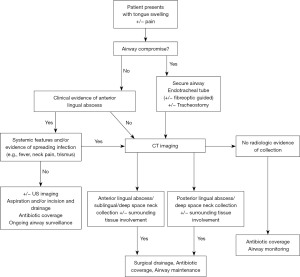
The cornerstone of lingual abscess management includes: (I) airway protection; (II) abscess drainage; and (III) antimicrobial therapy (1,19,27,38). Akin and Antoniades both highlight that patients presenting with signs of airway compromise including dyspnoea require immediate airway management and potentially tracheostomy (20,49). While aspiration might be recommended for abscess located anteriorly, incision and drainage may be required for posterior abscess, given access constraints. In some cases, patients underwent both aspiration and subsequent incision and drainage if resolution was not achieved with one management technique (18,20,30,32,33,36,42). We did not identify any differences in surgical approach between an anterior and posterior lingual abscess treatment using incision and drainage (67.4% vs. 51.9%, P=0.187, Table 3). The method of drainage may vary depending on the resources and clinical expertise. Only one reported mortality outcome was reported by Schweigert and colleagues which was attributed to a missed posterior lingual abscess (5). No significant difference was seen in morbidity or mortality rates, however more data is needed to ascertain any true correlation of increased risk based on location. For management, we recommend incision and drainage under general anaesthetic for posterior lingual abscess due to high risk of airway compromise (Figure 3). Clinician experience, patient co-morbidities and presences of systemic systems may also guide imaging and drainage decisions.
The literature reports empirical treatments should be commenced with broad-spectrum antibiotics typically covering the most common offending organisms within the oral cavity (Streptococcus spp, Staphylococcus spp, anaerobes and gram-negative) (2,21,41,42,54). Native oral and oropharynx flora such as Streptococcus spp, Staphylococcus spp, Haemophilus spp, Fusobacterium spp, Bacteroides spp and anaerobes are commonly reported in cases of lingual abscesses (1,16,27,30,38,44). Table 4 outlines the pathogens isolated, with some rarer organisms reported such as Acinetobacter iwoffi and Klebsiella ozaenae. In this review cases reported as mixed oral flora (5.6%), unknown (22.5%) or no growth (16.9%) account for just under 50%. This may be due to inaccurate reporting in the case report or series and the early administration of antibiotics affecting culture. Amoxicillin-clavulanic acid, clindamycin and ceftriaxone as multiple agents or combined with metronidazole were commonly prescribed across the literature (1,3,4,21,30). This review saw similar prescribing patterns however no significant difference was noted in prescribing based on location of abscess. To our knowledge, no specific guidelines on empirical antibiotic treatment regimens exist for lingual abscess, however empirical treatment for odontogenic infections recommends amoxicillin and clavulanate acid as a single preparation or metronidazole used in combination with a penicillin (amoxicillin or phenoxymethylpenicillin) (57). The duration of antibiotic therapy remains unclear and was poorly reported across the literature, it is recommended however that patients show improvement in 3–5 days on whatever regime has been prescribed (1). With almost a quarter of cases not reporting specific antibiotic, we recommend that antibiotic stewardship is in line with local health guidelines that appropriately manage odontogenic infections.
The reported mortality of lingual abscess is low (1.4%), with a single case of misdiagnosed posterior lingual abscess affecting the airway (1). With a comprehensive assessment and appropriate imaging in posterior abscesses allowing early diagnosis and definitive management (Figure 3), the morbidity and mortality should remain very low. The limitations of this review include the inconsistent reporting of data across the literature including biochemical markers on presentation, clinical observations, and other significant medical and/or surgical history.
Conclusions
Although anterior abscesses are largely a clinical diagnosis, posterior lingual abscesses are more likely to present with otalgia and/or sialorrhoea with CT imaging to guide involvement of surrounding structures and clarify the diagnosis. A comprehensive history and clinical assessment should accompany early airway assessment and protection as required. Thereafter the mainstays of management are the drainage of collections and antimicrobial therapy.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://www.theajo.com/article/view/10.21037/ajo-23-13/rc
Data Sharing Statement: Available at https://www.theajo.com/article/view/10.21037/ajo-23-13/dss
Peer Review File: Available at https://www.theajo.com/article/view/10.21037/ajo-23-13/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://www.theajo.com/article/view/10.21037/ajo-23-13/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Ethics approval was not required for this study. Because of the retrospective nature of the research, the requirement for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Srivanitchapoom C, Yata K. Lingual Abscess: Predisposing Factors, Pathophysiology, Clinical Manifestations, Diagnosis, and Management. Int J Otolaryngol 2018;2018:4504270. [Crossref] [PubMed]
- Carotenuto A, Menke B, Jolton J, et al. Recurrent Lingual Abscess in an Elderly Female With Bulbar Amyotrophic Lateral Sclerosis. Cureus 2022;14:e28280. [Crossref] [PubMed]
- Boon M, Pribitkin E, Spiegel J, et al. Lingual abscess from a grill cleaning brush bristle. Laryngoscope 2009;119:79-81. [Crossref] [PubMed]
- Sands M, Pepe J, Brown RB. Tongue abscess: case report and review. Clin Infect Dis 1993;16:133-5. [Crossref] [PubMed]
- Schweigert J, Christian R, Kemp WL. Challenges in the Diagnosis of a Posterior Lingual Abscess, a Potential Lethal Disorder: A Case Report and Review of the Literature. Am J Forensic Med Pathol 2020;41:64-6. [Crossref] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg 2021;88:105906. [Crossref] [PubMed]
- Joanna Briggs Institute. Critical Appraisal Tools. Available online: https://jbi.global/critical-appraisal-tools
- Jain HK, Bhatia PL. Lingual abscess. J Laryngol Otol 1970;84:637-41. [Crossref] [PubMed]
- Palestini M. Lingual abscess following traumatic penetration of a tooth into the tongue. Oral Surg Oral Med Oral Pathol 1981;52:485-6. [Crossref] [PubMed]
- Eames FA, Peters JC. CT findings in lingual abscess. J Comput Assist Tomogr 1983;7:544. [Crossref] [PubMed]
- Leggett JM. Use of ultrasound in the management of acute lingual swelling. J Laryngol Otol 1987;101:1312-4. [Crossref] [PubMed]
- Roberge RJ, Fowler RM 4th, Mayer NM. Glossal abscess. Am J Emerg Med 1989;7:406-8. [Crossref] [PubMed]
- Renehan A, Morton M. Acute enlargement of the tongue. Br J Oral Maxillofac Surg 1993;31:321-4. [Crossref] [PubMed]
- Hehar SS, Johnson IJ, Jones NS. Glossal abscess presenting as unilateral tongue swelling. J Laryngol Otol 1996;110:389-90. [Crossref] [PubMed]
- Jungell P, Asikainen S, Kuikka A, et al. Acute tongue abscess: report of two cases. Int J Oral Maxillofac Surg 1996;25:308-10. [Crossref] [PubMed]
- Muñoz A, Ballesteros AI, Brandariz Castelo JA. Primary lingual abscess presenting as acute swelling of the tongue obstructing the upper airway: diagnosis with MR. AJNR Am J Neuroradiol 1998;19:496-8. [PubMed]
- Olsen JC. Lingual abscess secondary to body piercing. J Emerg Med 2001;20:409. [Crossref] [PubMed]
- Brook I. Recovery of anaerobic bacteria from a glossal abscess in an adolescent. Pediatr Emerg Care 2002;18:358-9. [Crossref] [PubMed]
- Eviatar E, Pitaro K, Segal S, et al. Lingual abscess: secondary to follicular tonsillitis. Otolaryngol Head Neck Surg 2004;131:558-9. [Crossref] [PubMed]
- Antoniades K, Hadjipetrou L, Antoniades V, et al. Acute tongue abscess. Report of three cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2004;97:570-3. [Crossref] [PubMed]
- Balatsouras DG, Eliopoulos PN, Kaberos AC. Lingual abscess: diagnosis and treatment. Head Neck 2004;26:550-4. [Crossref] [PubMed]
- de Waal P, Prescott CA. More than a mouthful. S Afr Med J 2004;94:347-8. [PubMed]
- Kim HJ, Lee BJ, Kim SJ, et al. Tongue abscess mimicking neoplasia. AJNR Am J Neuroradiol 2006;27:2202-3. [PubMed]
- Kiroglu AF, Cankaya H, Kiris M. Lingual abscess in two children. Int J Pediatr Otorhinolaryngol Extra 2006;1:12-4. [Crossref]
- Nariai Y, Yanai C, Kondo S, et al. A fish bone in the tongue: a report of a case. Asian Journal of Oral and Maxillofacial Surgery 2010;22:30-2. [Crossref]
- Tajudeen BA, Lanson BG, Roehm PC. Glossal abscess as a complication of tongue-base suspension surgery. Ear Nose Throat J 2011;90:E15-7. [Crossref] [PubMed]
- Vellin JF, Crestani S, Saroul N, et al. Acute abscess of the base of the tongue: a rare but important emergency. J Emerg Med 2011;41:e107-10. [Crossref] [PubMed]
- Byahatti SM, Ingafou MSH. Lingual abscess- a rarity. J Clin Exp Dent 2011;3:e162-5. [Crossref]
- Veloo AC, Schepers RH, Welling GW, et al. Assessment of the microbiota of a mixed infection of the tongue using phenotypic and genotypic methods simultaneously and a review of the literature. Anaerobe 2011;17:47-51. [Crossref] [PubMed]
- Harrington AT, Hsia JC, Mendez E, et al. A lingual abscess caused by Streptococcus intermedius. J Med Microbiol 2012;61:590-2. [Crossref] [PubMed]
- Pallagatti S, Sheikh S, Kaur A, et al. Tongue abscess: a rare clinical entity. J Investig Clin Dent 2012;3:240-3. [Crossref] [PubMed]
- Sánchez Barrueco Á, Melchor Díaz MA, Jiménez Huerta I, et al. Recurrent lingual abscess. Acta Otorrinolaringol Esp 2012;63:318-20. [Crossref] [PubMed]
- Kikidis D, Marinakis K, Sengas J, et al. Lingual abscess in a psychiatric patient: a case report. Case Rep Med 2012;2012:194292. [Crossref] [PubMed]
- Solomon DM, Hahn B. Lingual abscess. J Emerg Med 2012;43:e53-4. [Crossref] [PubMed]
- Kulkarni CD, Verma AK, Kanaujia R. A rare case of hemilingual abscess in a 17-year-old girl: the ease of ultrasound and the advantage of MRI. Jpn J Radiol 2013;31:491-5. [Crossref] [PubMed]
- Burnham RMC, Hanu-Cernat L. Glossal abscesses–a rare presentation in the oral surgery world. Oral Surgery 2013;6:22-4. [Crossref]
- Varghese L, Agarwal P, Rupa V. Unusual complication of dental extraction: lingual abscess. Indian J Dent Res 2013;24:772-4. [Crossref] [PubMed]
- Kettaneh N, Williamson K. Spontaneous lingual abscess in an immunocompromised patient. Am J Emerg Med 2014;32:492.e1-2. [Crossref] [PubMed]
- Coughlin AM, Baugh RF, Pine HS. Lingual tonsil abscess with parapharyngeal extension: a case report. Ear Nose Throat J 2014;93:E7-8. [Crossref] [PubMed]
- Ozgur GT, Akdogan MV, Unler GK, et al. A rare cause of acute Dysphagia: abscess of the base of the tongue. Case Rep Gastrointest Med 2015;2015:431738. [Crossref] [PubMed]
- Lefler JE, Masullo LN. Lingual Abscess in the Setting of Recent Periodontal Antibiotic Injections. J Emerg Med 2016;51:454-6. [Crossref] [PubMed]
- Pandey MK, Srivastava A, Kushwaha R. Tongue is an unusual site of abscess development-an experience of two cases. International Journal of Medicine & Health Research 2016;2:1-4.
- Kuge R, Komori K, Miyama S. Recurrent Lingual Abscess in a Child. Pediatr Infect Dis J 2017;36:694-5. [Crossref] [PubMed]
- Bekele K, Markos D. Lingual abscess: a case report. Int Med Case Rep J 2017;10:285-7. [Crossref] [PubMed]
- Al-Anee AM, Asmael HM. The First Patient Report of Tongue Abscess Among Iraqi Population. J Craniofac Surg 2018;29:e243-5. [Crossref] [PubMed]
- Gama R, Ribeiro L, Domingues B, et al. Tongue abscess causing a septic shock: an unusual complication of a rare entity. Acta Otorrinolaringol Gallega 2018;11:90-6.
- Potigailo V, Weinsheim T. Base of the tongue abscess: An uncommon entity. Vis J Emerg Med 2018;10:7-8. [Crossref]
- Tewari N, Prakash Mathur V, Rajwar A, et al. 940-nm Diode Laser-Assisted Management of Tongue Abscess. J Dent Child (Chic) 2018;85:147-50. [PubMed]
- Akin V, Sivrice ME, Kumbul YÇ. A rare surgical emergency: lingual abscess. KBB ve BBC Dergisi 2020;28:317-20. [Crossref]
- Araidy S, Alkeesh K, Joachim MV, et al. Tongue abscess: a rare condition. A report of two cases and a summary of 66 reported cases. J Clin Case Rep Rev 2020;3:155.
- Mesolella M, Allosso S, Iorio B, et al. Clinical and Diagnostic Aspect of Tongue Abscess. Ear Nose Throat J 2021;100:1012S-4S. [Crossref] [PubMed]
- Wong CH, Chew SC. Tongue Abscess: Delayed Diagnosis of a Foreign Body in the Tongue. Clin Med Rev Case Rep 2021;8:347.
- Bülbül E, Yurtseven A, Kaynakcı Bayram M, et al. A Different Use of Ultrasonography in Emergency Service; A Rare Sore Throat: Tongue Abscess. Journal of Anatolian Medical Research 2021;6:95-7.
- Mesfin T, Debele G, Seyoum K, et al. Tongue Abscess: A Case Report. Int Med Case Rep J 2022;15:769-72. [Crossref] [PubMed]
- Little CC, Filimonov A, Schwam ZG. Lingual abscess: A case report of a rare clinical entity. Otolaryngology Case Reports 2022;23:100411. [Crossref]
- Chow A. Deep neck space infections in adults. UpToDate. 2022. Available online: https://www.uptodate.com/contents/deep-neck-space-infections-in-adults/print#!
- Therapeutic Guidelines. Acute odontogenic infections. 2019. Available online: https://ccmsfiles.tg.org.au/s3/PDFs/pdf_dtg3_table13.9_AcuteOdontogenticInfections_v5.pdf
Cite this article as: Wilson Z, Diab J, Ashford B. Clinical decision making for anterior and posterior lingual abscess: a systematic review. Aust J Otolaryngol 2023;6:21.