
Endonasal thermal imaging in the assessment of nasal obstruction and airflow
Introduction
Traditionally, the perception of nasal obstruction was assumed to be inadequate airflow or the sensation of increased airflow resistance through the nostrils (1). However, surgical experience dictates that simply providing increased nasal airflow does not always overcome “nasal obstruction” and no airflow receptors have been described (2). Direct airflow analysis from respiratory efforts or airflow resistance (rhinomanometry) has been the primary method for objectifying the perception of nasal breathing (3,4). Nonetheless, objective tests that evaluate resistance, peak inspiratory flow, and cross-sectional area correlate poorly with the subjective perception of nasal breathing (5-7). Current evidence suggests that nasal breathing is a result of mucosal cooling of the sensory receptors across the nasal cavity during inspiration (8-10). Pilot studies suggest the direct contact measures of nasal temperature might better predict the patient-reported perception of nasal breathing than nasal resistance and cross-sectional area (10). However, such direct contact techniques are unlikely to be tolerated by patients in the routine clinical setting.
Activation of cold thermoreceptors (TRPM8) through a temperature gradient on the sensory terminals of the trigeminal nerve in the nasal mucosa is thought to provide a sense of nasal breathing. A prime example of this process is the activation of the TRPM8 receptor by menthol, which induces a sense of clear nasal breathing without altering airflow (2). The impairment of the TRPM8 receptor or trigeminal nerve function contributes to the sensation of nasal obstruction (2,11,12).
Various objective tests for trigeminal nerve function have been proposed to measure trigeminal sensation for predicting the perception of nasal obstruction (10,13-17), including intranasal mucosal temperature measurement. Miniaturized and infrared thermocouples have been employed to assess intranasal mucosal temperature (16-18). Both methods demonstrated an association between a low mucosal temperature and a subjectively greater perception of nasal breathing (16-18). Nevertheless, the outcome is inconsistent, possibly due to mucosal irritation from the sensor after contact (10). Additionally, it is challenging to position the contact sensor on the inferior turbinates or nasal septum (10). Non-contact thermal sensing, such as an infrared (IR) camera, may be preferable.
The IR camera is a non-contact and non-invasive method for remote temperature monitoring. It is fast and can cover a large area simultaneously. The pseudo-color-coded thermograms are easy to interpret, and the technique has no radiation effects, making it suitable for repeated use. Moreover, the IR camera can monitor dynamic temperature changes in real time (19). Recent pilot studies seek to establish nasal vestibular temperature monitoring with the IR smartphone thermal camera as an objective measure of perception of nasal breathing (20,21). However, the temperature measurement result from the IR smartphone thermal camera was a number without the thermal image that can evaluate the area across nasal cavities, such as inferior turbinate or internal valve area (20,21).
Radiometric thermal imaging allows temperature data to be captured in each picture pixel. As a result, the camera can report the whole image with analyzed temperature data in a radiometric thermogram, producing quantitative results. The data was required to prove that intranasal mucosal temperature can be measured with the endonasal thermal image by the IR radiometric thermal camera. Consequently, this study was designed to develop the endonasal thermal image of the nasal passage to identify the association between intranasal mucosal temperature, subjective perception of nasal breathing, and objective measurement of nasal airflow in patients with nasal obstruction. We present this article in accordance with the STROBE reporting checklist (available at https://www.theajo.com/article/view/10.21037/ajo-23-20/rc).
Methods
Study design
A cross-sectional analysis of a single cohort of consecutively recruited and clinically evaluated patients with nasal obstruction at a tertiary rhinology center was performed between August 2022 and February 2023. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Macquarie University and St Vincent’s Hospital Human Research Ethics Committee (2018/ETH00733), and informed consent was taken from all individual participants.
Study population
Adult patients (>18 years) with primary symptoms of nasal obstruction/blockage/congestion were included. Patients with prior septoplasty and/or turbinate reduction, recent physical exertion (<30 min prior to assessment), recent hot or cold food and beverage intake (<30 min prior to assessment), history of anxiety and hyper-ventilatory disorders, history of thermal dysregulation disorders that are likely to impair normal thermal control of the body (i.e., thyroid disorders) or recent use of decongestant within 24 hours were excluded from the study. Patients were in a controlled 22 ℃ environment for at least 15 minutes before assessment.
Method of assessment for subjective perception of nasal breathing
The Nasal Obstruction Symptom Evaluation (NOSE) scale and perception of nasal obstruction on a visual analog scale (VAS) were completed to assess the subjective perception of nasal breathing. The NOSE scale was composed of five questions concerning the severity of nasal obstruction. Each item is evaluated using a Likert scale from 0 = not a problem to 4 = severe problem, summarized, and then multiplied by 5, for a total final NOSE score range between 0 and 100. Higher NOSE scores reflect greater severity of self-reported nasal obstruction (22). Patients also rated their subjective perception of nasal obstruction on a VAS of 0 to 100 mm (0 mm representing no obstruction and 100 mm representing complete obstruction). Patients were asked to cover one nostril and evaluate their ability to breathe through the uncovered nostril. The VAS score was used primarily to assess instantaneous patency of the nasal airway at the time of measurement, as opposed to the NOSE score, which assessed nasal obstruction over the past month (10).
Method of assessment for objective measurement of nasal airflow
Four-phase active anterior rhinomanometry (AAR) was performed at the international standard of 150 Pa using an NR6 Rhinomanometer (GM Instruments, Bristol, UK) to assess the objective measurement of nasal airflow. Measurements were taken after resting for at least 15 min in a climate-controlled room (22 ℃) and with the patient seated. An anaesthetic mask was held airtight around the nose, with the nostril contralateral to the testing side sealed with a nasal foam plug. The patient was instructed to breathe normally through the nose with the mouth closed. The opposite side was then tested using the same method. At least two readings of nasal airway resistance (NAR) within 10% of each other were obtained on each side. NAR was calculated by representative reading for each side using NARIS software (GM Instruments) and reported in Pa/cm3/s.
Intranasal mucosal temperature measurement
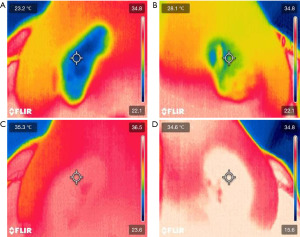
The VSC-IR21 probe with 19 mm round forward viewing tip connected to FILR VS290 infrared thermal videoscope kits (Teledyne FLIR, Wilsonville, Oregon) was placed approximately 1.5 cm from the subject’s nostril, similar to a basal view rhinoplasty photograph. The probe cable was attached to the adjustable microphone stand for image stabilization. Two disposable tongue depressors with a 1.5-cm distance marker from one edge were tied to the tip of the probe with a rubber band on both sides to ensure constant distance among the patients. The tip of tongue depressor that was near the midline was placed at the columella of the nose (Figure 1). The intranasal mucosal temperature presented on the endonasal thermal image of the nasal passage during a normal respiratory cycle at mid-inspiration temperature (InT) and mid-expiration temperature (ExT) was recorded using the single shot recording mode (Figure 2). The patient was instructed to breathe normally through the nose with the mouth closed.
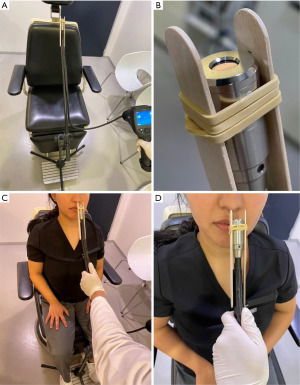
Measurement protocol
The intranasal mucosal temperature assessments were conducted by a single rhinologist utilizing a consistent protocol. All patients were measured with the following protocol. Patients spent at least 15 min acclimating in a climate-controlled room (22 ℃) before measurements. During this time, demographic data were obtained, including age and gender, and patients were administered the NOSE and pre-decongest VAS (VASpre) questionnaire. Next, the patient was seated in a chair for pre-decongest NAR (NARpre) measurement. Then, pre-decongest intranasal mucosal temperature measurement during a normal respiratory cycle at mid-inspiration (InTpre) and mid-expiration (ExTpre) was recorded using the single shot recording mode for three cycles per nostril. The measurement started with the left side first. Patients were sprayed with a topical decongestant, 0.05% oxymetazoline, 0.3 mL (3 sprays of 0.1 mL) per side. All patients waited 30 min before re-evaluating for post-decongestion measurements. The post-decongestion measurements comprised post-decongest VAS (VASpost) questionnaire, post-decongest NAR (NARpost), and post-decongest intranasal mucosal temperature measurement (InTpost and ExTpost).
Endonasal thermal image of the nasal passage
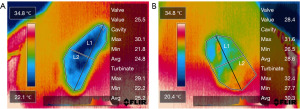
The FLIR thermal studio pro software (Teledyne FLIR, Wilsonville, Oregon) was used to edit thermal endonasal images of the nasal passage. The internal nasal valve area, nasal cavity area, and inferior turbinate area were defined in all thermal images. Two lines must be obtained to differentiate between nasal cavity and inferior turbinate area. The first line was the vertical-oblique line drawn from the highest to the lowest points of the thermal endonasal image. The second line, the horizontal-oblique line, was drawn from the lateral to medial borders of the thermal endonasal image perpendicularly through the midpoint of the first line. The area above the horizontal-oblique line was defined as the nasal cavity area, whereas the area below the horizontal-oblique line was defined as the inferior turbinate area. Then, the highest point of the vertical-oblique line was defined as the internasal nasal valve area (Figure 3). The intranasal mucosal temperature of the three areas was calculated and reported in ℃. There were 24 thermal images per patient (3 mid-inspiration and 3 mid-expiration images for each nostril in pre- and post-decongestion states).
Statistical analysis
ExT and InT of internal nasal valve area, nasal cavity area, inferior turbinate area was calculated from the mean of three cycles measurement for each nostril. Comparisons between ExT and InT (ΔExT-InT) of internal nasal valve area, nasal cavity area, inferior turbinate area, and overall airway (mean of three areas) in pre- and post-decongestion states were performed. Statistical analysis was conducted using SPSS Statistics version 28 (IBMCorp., Armonk, NY, USA). Q-Q plots were used for the normality test. Parametric results were expressed as mean ± standard deviation. Non-parametric results were expressed as median (interquartile range). Paired t-test and Wilcoxon signed-rank test were used for parametric and non-parametric continuous variables, respectively. The correlation coefficients were computed between intranasal mucosal temperature, subjective perception of nasal breathing, and objective measurement of nasal airflow. Pearson and Spearman’s rank correlation coefficients were used for parametric and non-parametric continuous variables, respectively. All P values were two-tailed, and a value of P<0.05 was considered statistically significant. The coefficient of variation was calculated to test the intraobserver variability.
Results
Of the 57 patients who were presented with primary symptoms of nasal obstruction, blockage, or congestion, 15 who had undergone prior septoplasty and/or turbinate reduction were excluded. Additionally, 9 patients with a history of anxiety were also excluded. Consequently, 33 patients (age 33.94±11.65 years, 39.4% female) were included in the study. Sixty-six nasal cavities were measured (left and right cavities for each participant). Fifteen patients were diagnosed with allergic rhinitis, and eighteen patients had non-allergic rhinitis. The data appeared to have a normal distribution when visually assessed using Q-Q plots. The NOSE scale was 59.85±26.65, VASpre was 57.03±28.35 mm, and NARpre was 0.67±0.62 Pa/cm3/s (normal <0.25 Pa/cm3/s). The ExTpre of the internal nasal valve area, nasal cavity area, inferior turbinate area, and overall airway were 31.32±2.19, 31.62±1.94, 32.18±1.81, and 31.71±1.95 ℃, respectively. The InTpre of the internal nasal valve area, nasal cavity area, inferior turbinate area, and overall airway were 27.25±2.32, 27.08±2.24, 27.65±2.35, and 27.32±2.23 ℃, respectively. ΔExT-InT of the internal nasal valve area, nasal cavity area, inferior turbinate area, and overall airway in pre- and post-decongestion states were presented in Table 1. ExT of all three areas and overall airway were higher than InT at both pre-decongestion and post-decongestion states. ΔExT-InT of the overall airway between left and right nasal cavities were not different in pre- (−0.23±0.51 ℃; P=0.66) and post-decongestion states (−0.51±0.42; P=0.23).
Table 1
| Intranasal mucosal temperature (℃) | ExT (N=66) | InT (N=66) | ΔExT-InT (N=66) | P value |
|---|---|---|---|---|
| Pre-decongestion | ||||
| Internal nasal valve | 31.32±2.19 | 27.25±2.32 | 4.08±2.24 | <0.001 |
| Nasal cavity | 31.62±1.94 | 27.08±2.24 | 4.54±2.14 | <0.001 |
| Inferior turbinate | 32.18±1.81 | 27.65±2.35 | 4.54±2.15 | <0.001 |
| Overall airway | 31.71±1.95 | 27.32±2.23 | 4.39±2.08 | <0.001 |
| Post-decongestion | ||||
| Internal nasal valve | 30.45±2.28 | 27.01±2.59 | 3.44±1.90 | <0.001 |
| Nasal cavity | 30.82±1.88 | 26.73±2.29 | 4.09±1.76 | <0.001 |
| Inferior turbinate | 31.36±1.71 | 27.16±2.32 | 4.20±1.76 | <0.001 |
| Overall airway | 30.88±1.92 | 26.97±2.37 | 3.91±1.73 | <0.001 |
Data are presented as mean ± standard deviation. ExT, mid-expiration temperature; InT, mid-inspiration temperature; pre, pre-decongestion; post, post-decongestion; SD, standard deviation.
Influence of nasal decongestion
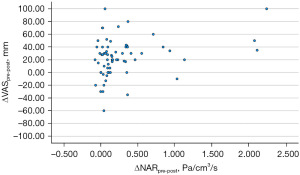
After decongestion, both VAS and NAR improved (57.03±28.35 vs. 33.30±24.16 mm, P<0.001; 0.67±0.62 vs. 0.38±0.23 Pa/cm3/s, P<0.001; respectively) (Table 2). Correlation analysis comparing ΔVASpre-post to ΔNARpre-post was performed. ΔVASpre-post had a statistically significant correlation with ΔNARpre-post (Pearson r=0.3; 95% CI: 0.06–0.5; P=0.014) (Figure 4).
Table 2
| Parameters | Pre (SD) (N=66) | Post (SD) (N=66) | ΔPre-Post (SD) (N=66) | P value |
|---|---|---|---|---|
| VAS (mm) | 57.03±28.35 | 33.30±24.16 | 23.73±29.66 | <0.001 |
| NAR (Pa/cm3/s) | 0.67±0.62 | 0.38±0.23 | 0.29±0.48 | <0.001 |
Data are presented as mean ± standard deviation. Pre, pre-decongestion; Post, post-decongestion; VAS, visual analog scale; SD, standard deviation; NAR, nasal airway resistant.
ΔExTpre-post and ΔInTpre-post of the internal nasal valve area, nasal cavity area, inferior turbinate area, and overall airway were presented in Table 3. ExTpost of three areas and overall airway were lower than ExTpre. However, ΔInTpre-post were not different. ΔExT-InTpre-post of the nasal cavity area, inferior turbinate area, and overall airway were also not different, except ΔExT-InTpre-post of the internal nasal valve area, which was statistically different (0.64±2.33 ℃; P=0.03). The coefficient of variation of ΔExT-InT of overall airway in pre- and post-decongestion states were 47.4% and 44.3%, respectively. There are no undesired reactions of the intranasal mucosal temperature measurements due to the non-contact measurement technique by IR camera.
Table 3
| Parameters (℃) | Pre (SD) (N=66) | Post (SD) (N=66) | ΔPre-Post (SD) (N=66) | P value |
|---|---|---|---|---|
| Internal nasal valve | ||||
| ExT | 31.32±2.19 | 30.45±2.28 | 0.87±2.04 | <0.001 |
| InT | 27.25±2.32 | 27.01±2.59 | 0.24±2.17 | 0.374 |
| ΔExT-InT | 4.08±2.24 | 3.44±1.90 | 0.64±2.33 | 0.03 |
| Nasal cavity | ||||
| ExT | 31.62±1.94 | 30.82±1.88 | 0.81±1.82 | <0.001 |
| InT | 27.08±2.24 | 26.73±2.29 | 0.35±2.10 | 0.183 |
| ΔExT-InT | 4.54±2.14 | 4.09±1.76 | 0.46±2.04 | 0.07 |
| Inferior turbinate | ||||
| ExT | 32.18±1.81 | 31.36±1.71 | 0.82±1.73 | <0.001 |
| InT | 27.65±2.35 | 27.16±2.32 | 0.48±2.13 | 0.07 |
| ΔExT-InT | 4.54±2.15 | 4.20±1.76 | 0.34±1.99 | 0.173 |
| Overall airway | ||||
| ExT | 31.71±1.95 | 30.88±1.92 | 0.83±1.82 | <0.001 |
| InT | 27.32±2.23 | 26.97±2.37 | 0.36±2.07 | 0.167 |
| ΔExT-InT | 4.39±2.08 | 3.91±1.73 | 0.48±2.01 | 0.059 |
Data are presented as mean ± standard deviation. ExT, mid-expiration temperature; InT, mid-inspiration temperature; Pre, pre-decongestion; Post, post-decongestion; SD, standard deviation.
Association of intranasal mucosal temperature with symptoms and airway resistance
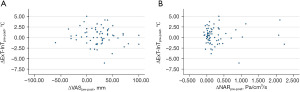
Correlation analysis comparing intranasal mucosal temperature (ΔExT-InT), subjective perception of nasal breathing (NOSE and VAS), and objective measurement of nasal airflow (NAR) of all areas at both pre- and post-decongestion states were performed. No correlations were found between intranasal mucosal temperature and subjective perception of nasal breathing (ΔExT-InT of overall airway and VAS pre-decongestion: Pearson r=−0.06; 95% CI: −0.31 to 0.19; P=0.612, ΔExT-InT of overall airway and NOSE pre-decongestion: Pearson r=−0.17; 95% CI: −0.42 to 0.07; P=0.165, ΔExT-InT of overall airway and VAS post-decongestion: Pearson r=−0.20; 95% CI: −0.44 to 0.05; P=0.109, ΔExT-InT of overall airway and NOSE post-decongestion: Pearson r=0.19; 95% CI: −0.06 to 0.43; P=0.138), and intranasal mucosal temperature and objective measurement of nasal airflow (ΔExT-InT of overall airway and NAR pre-decongestion: Pearson r=−0.02; 95% CI: −0.27 to 0.23; P=0.886, ΔExT-InT of overall airway and NAR post-decongestion: Pearson r=0.11; 95% CI: −0.14 to 0.36; P=0.385) at pre- and post-decongestion states. Additionally, correlation analysis was also performed between ΔExT-InTpre-post, ΔVASpre-post, and ΔNARpre-post at all areas. No correlations were found between ΔExT-InTpre-post and ΔVASpre-post (Pearson r=−0.12; 95% CI: −0.37 to 0.12; P=0.321), and between ΔExT-InTpre-post and ΔNARpre-post (Pearson r=0.03; 95% CI: −0.22 to 0.28; P=0.837) (Figure 5).
Discussion
Direct contact thermocouples have been used to measure the temperature of the intranasal mucosa (10,16,18). However, the development to use in clinical practice is complex, potentially with patient discomfort and mucosal irritation from a contact sensor. The practical non-contact sensor with an IR smartphone camera was described to record the nasal vestibular temperature in healthy subjects from a remote distance (20,21). However, sensitivity is weak as only one sample area from the middle of the nasal cavity area is taken. Clinically, the ideal IR radiometric thermal camera could create an endonasal thermal image with the ability to produce quantitative results from visualized structures in the nose, such as turbinates and septal mucosa.
This study proves that the IR radiometric thermal camera can measure the intranasal mucosal temperature in patients with nasal obstruction and present the result with the endonasal thermal image of the nasal passage. There is clearly a heterogenous distribution of temperatures within the nasal airway from Figure 3. ExT of three areas and overall airway were statistically higher than InT at pre-and post-decongestion states. Data of ExT and InT at pre-decongestion and post-decongestion states was in line with the previous studies that used the miniaturized and IR smartphone camera in healthy subjects (10,16,20). Clearer breathing during inspiration is associated with lower mucosal temperatures (10,16). The inspiratory nasal airflow evaporates water from the epithelial lining and activates trigeminal TRPM8 receptors through a temperature gradient. This activation generates neuronal depolarization to the brainstem respiratory center and cerebral cortex. The detection of temperature gradients is subsequently regarded as clear nasal breathing (2). On the other hand, blocked sensation during expiration is associated with higher temperatures. Throughout the expiration, the rise in temperature led to heating of the nasal mucosa by the warm air emanating from the lungs (16). This finding supports the hypothesis that mucosal cooling of the nasal mucosa is a crucial factor in the perception of nasal breathing.
The decreased VASpost and NARpost from pre-decongestion with statistically significant results confirmed that topical oxymetazoline reduced NAR and made the patients have clear nasal breathing. Additionally, ΔVASpre-post was significantly correlated with ΔNARpre-post. Although objective tests that evaluate resistance and cross-sectional area correlate poorly with the subjective perception of nasal breathing (5-7), some studies show more consistent correlation between rhinomanometry and subjective nasal obstruction (23,24). In the presence of a sensation of obstruction, the likelihood of correlation with objective tests is comparatively higher than in its absence. Furthermore, a stronger correlation has been observed between unilateral symptoms and objective measurements in contrast to bilateral symptoms and the cumulative mean cross-sectional areas or overall nasal resistance (25).
The present study shows that the ExTpost of three areas and overall airway were statistically lower than ExTpre after topical oxymetazoline. Oxymetazoline is the mixed α1- and α2-adrenoreceptor agonist that acts on α1-adrenoreceptor on the arteriolar side and α2-adrenoreceptor on the venular side in nasal mucosal vasculature causing the vasoconstriction effect (26). The reduction of mucosal blood flow from topical decongestant decreases the intranasal mucosal temperature (27). Moreover, diminished intranasal mucosal temperature could be attributed to greater radiant cooling from airflow from increased nasal cavity volume (27). Either mechanism produces mucosal cooling.
This study cannot demonstrate the correlation between intranasal mucosal temperature (ΔExT-InT, ΔExT-InTpre-post), subjective perception of nasal breathing (NOSE, VAS, and ΔVASpre-post), and objective measurement of nasal airflow (NAR and ΔNARpre-post). The temperature measurement technique in this study differed from the previous studies that show the correlation between intranasal mucosal temperature and subjective perception of nasal breathing or objective measurement of nasal airflow (10,17,18). Miniaturized thermocouples used in the previous study were inserted in the nasal cavity and positioned on the nasal mucosa. The accuracy may be better than the IR radiometric thermal camera that measured from outside the nose. Nevertheless, the problem with the contact sensor pertained to mucosal irritation and its inability to consistently demonstrate a correlation between mucosal temperature and VAS across the nasal cavity (10).
Additionally, the participants in this study differed from the previous literature (16-18,20). The correlation between intranasal mucosal temperature and subjective perception of nasal breathing or objective measurement of nasal airflow was demonstrated in healthy subjects (10,17,18). However, there was no data on the patients with nasal obstruction. In the present study, some cases had structural abnormality problems, such as severe nasal septal deviation or nasal valve collapse, that hindered the view of the structure inside the nose. The endonasal thermal image from the IR radiometric thermal camera with the probe outside the nasal cavity may not represent the exact intranasal mucosa. The temperature data was extracted from each picture pixel of the thermal image. If the thermal image cannot depict the precise structure inside the nose, the accuracy of the intranasal temperature may be discrepant.
This study confirms that the endonasal thermal image of the nasal passage can measure the intranasal mucosal temperature in patients with nasal obstruction. The temperature measurement technique from the IR radiometric thermal camera is a potential novel objective test for evaluating the perception of nasal breathing. The benefit of this technique is a non-invasive, non-radiation, and non-contact with straightforward technical instruction and interpretation. Additionally, it can be used repetitively and is suitable for dynamic temperature changes in nasal breathing.
However, it is clear that much more anatomical definition of the IR image is required as substantial heterogeneity of temperatures exists within the nasal airway. Current IR cameras don’t provide this and are too early for utilization in clinical practice. Moreover, the coefficient of variation of temperature measurement is high due to the limitation in thermal camera technology that cannot identify the precise structure inside the nose. Further studies require the IR radiometric thermal camera with a 3-4 mm diameter probe, short focal range, and the ability to give a true thermal endonasal image.
Conclusions
Endonasal thermal imaging demonstrates significant heterogeneity of readings within the nasal airway but is sensitive to changes in intra-nasal vascularity and airflow. The potential for IR radiometric thermal cameras as a reliable method for measuring intranasal mucosal temperature in patients with nasal obstruction warrants further investigation.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://www.theajo.com/article/view/10.21037/ajo-23-20/rc
Data Sharing Statement: Available at https://www.theajo.com/article/view/10.21037/ajo-23-20/dss
Peer Review File: Available at https://www.theajo.com/article/view/10.21037/ajo-23-20/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://www.theajo.com/article/view/10.21037/ajo-23-20/coif). L.K. and R.G.C. serve as unpaid editorial board members of Australian Journal of Otolaryngology from January 2019 to December 2023. K.S. serves as unpaid editorial board members of Australian Journal of Otolaryngology from January 2020 to December 2023. R.J.H. serves as the Editor-in-Chief of Australian Journal of Otolaryngology. R.J.H. is consultant/advisory board with Medtronic, Novartis, Glaxo-Smith-Kline and Meda pharmaceuticals. He has been on the speakers’ bureau for Glaxo-Smith-Kline, AstraZeneca, Meda Pharmaceuticals and Seqirus. L.K. is on the speakers’ bureau for Mylan, and Seqirus Pharmaceuticals. J.R. has honoraria with Sanofi Aventis, Novartis, Mundipharma, GSK, and Stallergenes. R.G.C. is on the speakers’ burea for Medtronic, Seqirus, GSK and Viatris. K.S. received honoraria for speaking at symposia from Organon, Viatris, and Menarini. R.S. is a consultant for Medtronic and is on the speakers bureau for Meda Pharmaceuticals. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Macquarie University and St Vincent’s Hospital Human Research Ethics Committee (2018/ETH00733) and informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yepes-Nuñez JJ, Bartra J, Muñoz-Cano R, et al. Assessment of nasal obstruction: correlation between subjective and objective techniques. Allergol Immunopathol (Madr) 2013;41:397-401. [Crossref] [PubMed]
- Kanjanawasee D, Campbell RG, Rimmer J, et al. Empty Nose Syndrome Pathophysiology: A Systematic Review. Otolaryngol Head Neck Surg 2022;167:434-51. [Crossref] [PubMed]
- Clement PA, Gordts FStandardisation Committee on Objective Assessment of the Nasal Airway, IRS, and ERS. Consensus report on acoustic rhinometry and rhinomanometry. Rhinology 2005;43:169-79. [PubMed]
- Cole P, Roithmann R, Roth Y, et al. Measurement of airway patency. A manual for users of the Toronto systems and others interested in nasal patency measurement. Ann Otol Rhinol Laryngol Suppl 1997;171:1-23. [PubMed]
- Jones AS, Willatt DJ, Durham LM. Nasal airflow: resistance and sensation. J Laryngol Otol 1989;103:909-11. [Crossref] [PubMed]
- Reber M, Rahm F, Monnier P. The role of acoustic rhinometry in the pre- and postoperative evaluation of surgery for nasal obstruction. Rhinology 1998;36:184-7. [PubMed]
- Gungor A, Moinuddin R, Nelson RH, et al. Detection of the nasal cycle with acoustic rhinometry: techniques and applications. Otolaryngol Head Neck Surg 1999;120:238-47. [Crossref] [PubMed]
- Zhao K, Blacker K, Luo Y, et al. Perceiving nasal patency through mucosal cooling rather than air temperature or nasal resistance. PLoS One 2011;6:e24618. [Crossref] [PubMed]
- Zhao K, Jiang J, Blacker K, et al. Regional peak mucosal cooling predicts the perception of nasal patency. Laryngoscope 2014;124:589-95. [Crossref] [PubMed]
- Bailey RS, Casey KP, Pawar SS, et al. Correlation of Nasal Mucosal Temperature With Subjective Nasal Patency in Healthy Individuals. JAMA Facial Plast Surg 2017;19:46-52. [Crossref] [PubMed]
- Bischoff S, Poletti SC, Kunz S, et al. Trigeminal endonasal perception - an outcome predictor for septoplasty. Rhinology 2020;58:437-43. [Crossref] [PubMed]
- Saliba J, Fnais N, Tomaszewski M, et al. The role of trigeminal function in the sensation of nasal obstruction in chronic rhinosinusitis. Laryngoscope 2016;126:E174-8. [Crossref] [PubMed]
- Poletti SC, Hausold J, Herrmann A, et al. Topographical distribution of trigeminal receptor expression in the nasal cavity. Rhinology 2019;57:147-52. [Crossref] [PubMed]
- Frasnelli J, Heilmann S, Hummel T. Responsiveness of human nasal mucosa to trigeminal stimuli depends on the site of stimulation. Neurosci Lett 2004;362:65-9. [Crossref] [PubMed]
- Meusel T, Negoias S, Scheibe M, et al. Topographical differences in distribution and responsiveness of trigeminal sensitivity within the human nasal mucosa. Pain 2010;151:516-21. [Crossref] [PubMed]
- Lindemann J, Leiacker R, Rettinger G, et al. Nasal mucosal temperature during respiration. Clin Otolaryngol Allied Sci 2002;27:135-9. [Crossref] [PubMed]
- Willatt DJ, Jones AS. The role of the temperature of the nasal lining in the sensation of nasal patency. Clin Otolaryngol Allied Sci 1996;21:519-23. [Crossref] [PubMed]
- Lindemann J, Keck T, Scheithauer MO, et al. Nasal mucosal temperature in relation to nasal airflow as measured by rhinomanometry. Am J Rhinol 2007;21:46-9. [Crossref] [PubMed]
- Lahiri BB, Bagavathiappan S, Jayakumar T, et al. Medical applications of infrared thermography: A review. Infrared Phys Technol 2012;55:221-35. [Crossref] [PubMed]
- Davila RO, Flynn J, Sykes KJ, et al. Nasal Vestibular Temperature as an Objective Measurement of the Nasal Airway Utilizing a Smartphone Thermal Imager: Proof of Concept. Facial Plast Surg Aesthet Med 2021;23:395-6. [Crossref] [PubMed]
- Jiang S, Chan J, Stupak HD. The Use of Infrared Thermal Imaging to Determine Functional Nasal Adequacy: A Pilot Study. OTO Open 2021;5:2473974X211045958.
- Stewart MG, Witsell DL, Smith TL, et al. Development and validation of the Nasal Obstruction Symptom Evaluation (NOSE) scale. Otolaryngol Head Neck Surg 2004;130:157-63. [Crossref] [PubMed]
- Sipilä J, Suonpää J, Silvoniemi P, et al. Correlations between subjective sensation of nasal patency and rhinomanometry in both unilateral and total nasal assessment. ORL J Otorhinolaryngol Relat Spec 1995;57:260-3. [Crossref] [PubMed]
- Simola M, Malmberg H. Sensation of nasal airflow compared with nasal airway resistance in patients with rhinitis. Clin Otolaryngol Allied Sci 1997;22:260-2. [Crossref] [PubMed]
- André RF, Vuyk HD, Ahmed A, et al. Correlation between subjective and objective evaluation of the nasal airway. A systematic review of the highest level of evidence. Clin Otolaryngol 2009;34:518-25. [Crossref] [PubMed]
- Corboz MR, Rivelli MA, Varty L, et al. Pharmacological characterization of postjunctional alpha-adrenoceptors in human nasal mucosa. Am J Rhinol 2005;19:495-502. [Crossref] [PubMed]
- Lindemann J, Leiacker R, Rettinger G, et al. The effect of topical xylometazoline on the mucosal temperature of the nasal septum. Am J Rhinol 2002;16:229-34. [Crossref] [PubMed]
Cite this article as: Seresirikachorn K, Png LH, Do TQP, Kalish L, Campbell RG, Rimmer J, Alvarado R, Raji N, Choy C, Snidvongs K, Sacks R, Harvey RJ. Endonasal thermal imaging in the assessment of nasal obstruction and airflow. Aust J Otolaryngol 2023;6:26.


