
Glial choristoma of the temporal bone: a case series and literature review
Introduction
A choristoma is defined as the presence of histologically normal tissue beyond the confines of where it normally resides (1). It may also be referred to as ectopic or heterotopic tissue. In the head and neck region, glial choristomas principally occur in midline structures, such as the nasopharynx, oropharynx, palate, oral cavity and tongue (2). Choristomas in non-midline structures such as the temporal bone are uncommon and are mainly composed of salivary gland tissue (3).
Choristomas composed of glial tissue in the temporal bone are rare as only a handful of cases have been reported in the literature (1,4-17).
With respect to the temporal bone, glial choristoma must be differentiated from a meningocele or meningoencephalocele, which is a protrusion of the meninges and/or brain through a defect in the skull-base (18). It may also be misinterpreted as a cholesteatoma, teratoma, schwannoma or neurofibroma. Glial choristoma may be found incidentally on clinical examination (mass behind tympanic membrane), radiologically (soft tissue within the middle ear or temporal bone), or intraoperatively during surgery for infection or cholesteatoma. When symptomatic, glial choristoma may cause hearing loss, aural fullness, or tinnitus (4).
This article presents four cases of glial choristoma of the temporal bone and a review of the current literature. At the time of writing, we believe this to be the largest case series from a single surgeon in a tertiary neurotology centre. We present this article in accordance with the CARE reporting checklist (available at https://www.theajo.com/article/view/10.21037/ajo-23-9/rc).
Methods
Patients with histologically confirmed glial choristoma of the temporal bone were included in this retrospective case series. These patients were all referred into the neurotology practice by their primary care providers. The preoperative workup of each patient consisted of history, clinical examination, pure tone audiometry and imaging either with computed tomography (CT), magnetic resonance imaging (MRI) scanning or both. Data was obtained from the medical record and included: baseline demographics, symptomology, surgical approach, intraoperative findings and post-surgical outcome.
Regarding the literature review, an electronic search was performed for case reports and series about glial choristoma of the temporal bone via MEDLINE and Embase from their respective dates of inception up until November 2022. The electronic search was performed using a combination of keywords consisting of ‘glial’, ‘neuroglial’, ‘choristoma’ and ‘temporal’. The resulting articles were individually screened, the bibliographies were also searched for any further relevant articles. Non-English texts were excluded.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the Helsinki Declaration (as revised in 2013). The study was approved by the Gold Coast Health and Hospital Service Human Research Ethics Committee (LNR/2022/QGC/70168). Due to the low-risk nature of the reporting, the local research and ethics committee waived the need for informed consent.
Results
A total of four patients who presented between 2008 to 2022 were identified as having histologically confirmed glial choristoma of the temporal bone. We present their respective cases are below and in Table 1.
Table 1
| Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|
| Patient demographic | 27-year-old female | 32-year-old female | 55-year-old female | 56-year-old female |
| Presenting symptoms | Mild bilateral hearing loss and tinnitus. No balance disturbance, otalgia or otorrhoea | Right otalgia, mastoid tenderness and subjectively reduced hearing. No tinnitus or balance disturbance | Progressive hearing loss bilaterally. Intermittent humming tinnitus is more pronounced on right side. No vertigo, otalgia or otorrhoea | Mild left sided hearing loss. Bilateral intermittent ringing tinnitus, aural fullness and otorrhoea (more on left than right) |
| Otological background | Right MRM for attic cholesteatoma (15 years ago). Multiple sets of ventilation tubes in childhood | Right cortical mastoidectomy and insertion of ventilation tube 18 months prior | Nil significant | Adenotonsillectomy and multiple sets of ventilation tubes in childhood |
| Clinical examination | Adequate right MRM cavity and meatoplasty with no recidivism. Small attic cholesteatoma | Intact right tympanic membrane with acute otitis media (ventilation tube inserted at time). No subperiosteal swelling or abscess over mastoid | Bilateral superior osteomas. Right tympanic membrane was retracted and thickened. Left tympanic membrane normal | Left: generalised myringitis, central tympanic membrane retraction or perforation along with attic cholesteatoma |
| Right: retracted and immobile tympanic membrane | ||||
| Imaging | CT: left attic cholesteatoma and tegmen defect. Confirmed with MRI | CT: opacification of right mastoid cavity and middle ear space. No suggestion of cholesteatoma or tegmen defect | CT: dehiscence of right tegmen tympani and superior semicircular canal | CT: bilateral pars flaccida cholesteatoma with erosion of tegmen tympani |
| MRI: 2–3 mm tegmen defect with no temporal lobe herniation. No other soft tissue mass was seen | MRI: subtle herniation of the right temporal lobe | |||
| Intervention | Left combined approach tympanoplasty in 2 stages. The 3rd stage procedure converted into modified radical mastoidectomy | Right revision mastoidectomy and middle ear exploration | Exploratory right tympanotomy followed by relook combined approach tympanoplasty with repair of tegmen defect | Left combined approach tympanoplasty. Repair of tegmen defect |
| Outcome | No clinically evident signs of recurrence, author unaware of any problems 13 years postoperatively | No clinically evident signs of recurrence, author unaware of any problems 13 years postoperatively | Ongoing tinnitus consistent with semicircular canal dehiscence, well tolerated and managed conservatively. No clinical signs of choristoma recurrence 5 years postoperatively | No clinical signs of choristoma recurrence, author unaware of problems 1 year postoperatively |
MRM, modified radical mastoidectomy; CT, computed tomography; MRI, magnetic resonance imaging.
Our literature search yielded 13 case reports and 2 case series published about glial choristoma of the temporal bone, summarised in Table 2.
Table 2
| Case report/series | Patients | Symptoms/history | Location | Imaging | Tegmen dehiscence |
|---|---|---|---|---|---|
| McGregor 1994 (5) | 3 M | Right otalgia, previous meningitis | R ME | N/A | Yes |
| 36 M | Chronic right otitis media | R ME | N/A | Yes | |
| 65 M | Chronic otitis media and hearing loss | R ME | N/A | Yes | |
| Lee 2004 (6) | 50 M | Progressive hearing loss and otorrhoea of left ear | L ME | CT: soft tissue density occupying middle ear cavity and mastoid antrum | Yes |
| Farneti 2007 (7) | 74 M | Recurrent bilateral otitis media | L ME | CT: soft tissue mass occupying left epitympanum, aditus and antrum | No |
| Uğuz 2007 (8) | 61 M | Progressive left sided hearing loss and tinnitus | L ME | CT: soft tissue mass occupying left middle ear and mastoid | Yes |
| Aubry 2009 (9) | 66 F | Vertigo and tinnitus | R IAC | MRI: lesion in IAC with hypersignal on T1&2 weighted images | No |
| Martinez-Peñuela 2011 (10) | 61 M | Progressive right sided hearing loss | R ME | CT: soft tissue mass filling antrum and attic | Yes |
| 61 M | Left sided hypoacusia and tinnitus | L ME | CT: soft tissue mass in attic | Yes | |
| Shemanski 2013 (11) | 81 M | Left sided chronic otitis media, hearing loss, tinnitus and aural fullness | L ME | CT: opacification of left mastoid process MRI: fluid in middle ear and mastoid consistent with inflammatory tissue |
Yes |
| Wu 2013 (12) | 3 M | 10-day history of left otalgia | L ME | CT: non enhancing mass in middle ear MRI: non enhancing mass |
No |
| Rizk 2013 (13) | 31 F | Progressive left sided hearing loss | L IAC | MRI: non enhancing hypointense lesion on T1&2 in IAC | No |
| Dunham 2013 (14) | 7 M/0 F | Enlarging left lateral scalp mass over mastoid, causing EAC obstruction | L scalp (mastoid) | CT: soft tissue mass posterior to pinna with area of invagination into mastoid bone | No |
| Aghaghazvini 2016 (15) | 7 M/0 F | Gradual painless left sided facial swelling | L infratemporal fossa | US: solid cystic mass in parotid space | No |
| MRI: multilobulated solid cystic mass occupying infratemporal fossa | |||||
| Shim 2016 (4) | 63 F | Right sided hearing loss and aural fullness | R mastoid antrum | CT: soft tissue mass in right mastoid antrum with calcification | Yes |
| AlZubaidi 2018 (16) | 68 F | Bilateral chronic otitis media with conductive hearing loss | L mastoid | MRI: left middle ear mass without continuity with CNS | No |
| Quatre 2020 (1) | 63 M | Progressive right sided hearing loss and tinnitus | R ME | CT: soft tissue mass occupying the right middle ear | Yes |
| Pöhlmann 2021 (17) | 59 F | Progressive left sided hearing loss | L ME | CT: middle ear mass, filling epitympanum | Yes |
M, male; F, female; L, left; R, right; N/A, not available; ME, middle ear; CT, computed tomography; IAC, internal auditory canal; MRI, magnetic resonance imaging; EAC, external auditory canal; US, ultrasound; CNS, central nervous system.
Case 1
A 27-year-old female was referred with a left attic cholesteatoma in the context of previous right sided disease 15 years ago that was surgically managed. She had left sided hearing loss and tinnitus, there was no otalgia or balance disturbance. Clinical examination revealed a small left attic cholesteatoma with erosion of the scutum and long process of incus.
High resolution CT (HRCT) of the temporal bones was performed, demonstrating soft tissue opacification in the attic and sinus tympani. There was also a suggestion of a tegmen defect. MRI of temporal bones demonstrated a small (5 mm) mass in the epitympanum separate from the tegmen tympani defect. This lesion showed no restricted diffusion and was isointense to adjacent temporal cortex on T1 and T2 weighted images. Peripheral enhancement was seen on post contrast images (Figure 1).
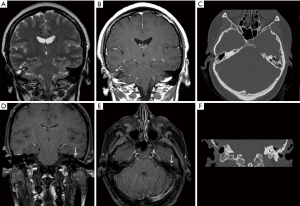
She underwent a combined approach tympanoplasty with complete macroscopic removal of the cholesteatoma including incus and head of malleus. A small, orange, spherical lesion was identified along the tegmen posterior to the actual tegmen defect (approximately 12 mm separation) and sent for histological analysis. There were no postoperative problems.
The lesion was examined histologically, and showed fibrous and glial tissue, and was positive for glial fibrillary acidic protein (GFAP) on immunohistochemistry (IHC), consistent with glial choristoma of the temporal bone.
Given ongoing clinical recurrence she underwent a 2nd and 3rd stage operation. She has subsequently healed with no evidence of recurrence and the author is unaware of any problems 13 years later.
Case 2
A 32-year-old female presented with a 1-month history of right otalgia, mastoid region tenderness and right hearing loss. There was no otorrhoea, tinnitus nor balance disturbance. Her otological background was significant for a recent right cortical mastoidectomy done for similar reasons.
Clinical examination revealed an intact right tympanic membrane with acute otitis media, with no subperiosteal swelling nor abscess over the mastoid. She was treated initially with insertion of a ventilation tube and antibiotics. Her symptoms improved initially, then relapsed and deteriorated.
HRCT and MRI of the temporal bones were performed. The CT scan demonstrated partial opacification of the mastoid cavity and middle ear space suggestive of mucosal oedema and fluid, with no suggestion of cholesteatoma nor tegmen defect. The MRI demonstrated a 2-3 mm tegmen defect with no brain herniation. T2W images showed a small focus of high signal in the temporal lobe with minimal enhancement on T1W coronal images. On these images, the tegmen defect was identified but the soft tissues mass was not identified.
A right revision mastoidectomy and middle ear exploration were performed. Prior to any drilling, a small tegmen defect was identified, with pulsatile dura on view (covered by inflamed mucosa) with no cerebrospinal fluid (CSF) leak. The tissue sent for histology was taken from a different site to this area, separated by approximately 10 mm.
The mastoid tissue sent for histology showed glial tissue admixed with fibrovascular tissue and a fibrous pseudocapsule. Given that this tissue was separate from the tegmen defect, it was felt to represent a glial choristoma, rather than encephalocele or brain herniation. She had an uneventful postoperative course and as far as the author is aware, she remains trouble-free more than 10 years later.
Case 3
A 55-year-old female presented with progressive hearing loss over 5 years with associated tinnitus and aural fullness on the right side. There was no significant otological history. Clinical examination of the right ear demonstrated a small osteoma along with a retracted and thickened tympanic membrane, which was mobile with Valsalva manoeuvre. The remainder of her examination was unremarkable.
An exploratory right tympanotomy was performed, a thick granular lesion overlying the ossicles was encountered, removed and sent for histological analysis. Her postoperative course was unremarkable. Histology of the lesion demonstrated a glial choristoma with slides showing neuronal and glial cells, and GFAP and S100 positive on immunohistochemistry.
Given the histology results, a CT and MRI of the temporal bones were performed. The CT showed evidence of dehiscence of the anterior portion of the right tegmen tympani with fluid filled density in the middle ear suggestive of a possible direct connection. The MRI demonstrated a subtle herniation of the right temporal lobe and diffusion weighted imaging (DWI) was within normal limits.
A combined approach tympanoplasty was performed. Lobulated soft tissue presumed to be glial choristoma was seen in the antrum, aditus and epitympanum surrounding the ossicles which were otherwise intact and mobile. The choristoma was removed along with the incus and head of the malleus. A >10 mm tegmen defect was encountered along with a mild CSF leak which was repaired. Her right ear has healed well and to date, there are no signs of recurrence of the glial choristoma.
Case 4
A 56-year-old female was referred for chronic left sided ear issues which included a mild hearing loss, aural fullness, otorrhoea and bilateral intermittent tinnitus. Otological history significant for 10 previous sets of ventilation tubes.
Clinical examination of the left ear demonstrated generalised myringitis, with a central tympanic membrane retraction or perforation and a small attic cholesteatoma. The remainder of her examination was unremarkable. A CT scan showed a bilateral pars flaccida cholesteatoma centred on Prussak’s space with erosion of the tegmen tympani and confluent extension into the anterior mastoid cells.
She underwent a left combined approach tympanoplasty. A small left posterosuperior pars tensa cholesteatoma was encountered and removed. Polypoid lesions were also noted in the middle ear and mastoid, which were removed and sent for histopathology. As expected from our preoperative imaging, the tegmen dehiscence was exposed and repaired with DuraGen and a temporalis muscle flap, there was a mild CSF leak intra-operatively.
Histological analysis of the lesions showed neuroglial tissue with associated areas of necrosis surrounded by granulomatous inflammation. Her postoperative course was uneventful and she will undergo a second stage procedure in the near future.
Discussion
Literature review
The first described case of extracranial heterotopic glial tissue was in the dorsal surface of the cervical spinal cord in 1907 by Wolbach (19). In 1994, McGregor et al. (5) reported 3 cases of glial choristoma in the temporal bone, all of which were associated with cholesteatoma. In these cases, the heterotopic glial tissue was considered to be a result of local bony destruction secondary to cholesteatoma, otitis media, meningitis or previous surgery.
Interestingly, glial choristomas involving the temporal bone have also been reported in infants (4,15); however, they were in different regions: occupying the infratemporal fossa or externally on the mastoid with invagination into bone. Both infants did not have an identified tegmen defect.
Including our own series of 4 patients, we have identified a total of 22 patients in the literature. Of the 22 patients, 15 had a tegmen defect (68.2%) identified either preoperatively with imaging or intraoperatively during exploration.
Clinical features
A tegmen defect was present in all cases and glial choristomas were either adherent to the tegmen tympani or ossicular chain but were separate from the tegmen defect by 10–12 mm (Figure 2). There was no herniation of meninges nor brain tissue. A CSF leak was encountered in two of the cases (cases 3 and 4) in the process of removing granulations from around the choristomas. No patients developed any CSF leaks prior to surgery.
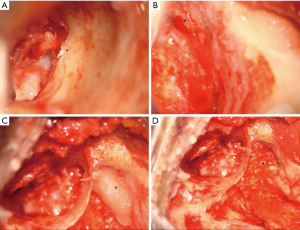
Radiological features
Our patients underwent both HRCT and MRI of the temporal bones. In the setting of glial choristoma, CT and MRI are complementary imaging modalities, the CT useful in depicting the bony tegmen defect and MRI in determining the soft tissue mass and excluding meningo-encephalocele.
Case 2 highlights the importance of utilising complementary imaging as the initial CT did not suggest cholesteatoma or tegmen defect, whereas a 2–3 mm defect was later identified in a following MRI.
In general, our series of MRI images of the lesions showed no restricted diffusion and isointensity to adjacent tissue on T1 and T2 weighted images (Figure 1). Similar features have also been described by Wu et al. (12) and Rizk et al. (13).
The radiological features in conjunction with the small size of the lesions render the diagnosis of glial choristoma difficult preoperatively.
Histological features
Glial choristoma appears as mature brain tissue with neuronal and glial cells, sometimes incorporating choroid plexus and ependymal or leptomeningeal cells without evidence of chronic inflammatory cells (20). Our cases represent a mixture of glial tissue comprising astrocytic glial cells and some neurones within a fibrillary eosinophilic background (composed of astrocytic cell processes) with varied degrees of degenerative fibrosis. There are also some meningeal cells present (Figure 3). They were also S100 and GFAP positive on immunohistochemistry. Similar to our results, there have been no reports of cellular atypia or atypical mitosis in the literature (7,12,21).

The differential diagnoses include glioma, ganglioneuroma, meningioma, neuroma, and schwannoma (1,7), although these can usually be distinguished histologically. Interestingly, there are no significant histologic differences between heterotopic brain tissue and encephalocele, as they are both characterised by variable proportions of glial and neural cells (22). Therefore, radiological and intraoperative findings are required to differentiate the two.
Pathogenesis
There are two main theories of pathogenesis: rudimentary meningoencephalic herniation or residual neural crest tissue (1,23).
Meningoencephaloceles may either be congenital due to arrested closure of bone of the skull base during development, or acquired later in life secondary to trauma, surgery, neoplasm or inflammation (17,24). If the connecting stalk atrophies, the glial tissue would remain in an ectopic location, with no intervening glial tissue. This theory would seem most feasible if the glial choristoma was adjacent or attached to the exposed dura of the bony defect.
The residual neural crest theory relates to maldevelopment of neuroectodermal cells at a distant location which may form mature brain tissue with no connection to the brain (7). This would not necessitate a bony defect to be present, as in the case of meningoencephalocele. Glavis-Bloom et al. described a noteworthy case of a glial choristoma present in the foot of an otherwise healthy 13-month-old female (23). However, this theory would be better supported by an intact tegmen tympani, which was not encountered in any of our patients. To add a further element of complexity, a neuroectodermal remnant preventing the bony closure of the skull base may mimic a sequestered meningoencephalocele (25).
All four cases described in our series had a well-encapsulated lesion which was separate to rather than attached to the dura overlying the tegmen defect. Our favoured explanation is that a small defect in the tegmen tympani (whether congenital or acquired, probably congenital) has permitted the herniation of glial tissue. It is therefore possible that patients with congenital tegmen defects are more likely to develop this condition.
Subsequent spontaneous retraction of most of the herniated tissue and partial closure of the defect has then occurred, leaving a small glial choristoma remnant in the middle ear or mastoid, adjacent to the remaining tegmen defect.
Irrespective of the pathogenesis, the treatment remains the same regardless of causation, usually with removal of glial tissue and repair of bone skull base defect and CSF leak if present.
Conclusions
Glial choristoma of the temporal bone is a rare condition which may be found incidentally or in association with infection or cholesteatoma. Whether from rudimentary meningoencephalocele or residual neuroectodermal tissue, treatment involves removal of the glial choristoma and repair of the bony skull base defect.
This condition’s actual prevalence is likely higher in the general population as most cases are asymptomatic. In our case series, glial choristomas exist as an incidental finding during surgery, suggesting untreated patients could remain symptom-free unless they require surgical exploration for a separate otological pathology.
Prior to operative intervention, patients should be evaluated with CT and MRI as it is important to differentiate a discrete lesion from a meningocele or meningoencephalocele as this may require additional surgical measures and may pose an increased risk of meningitis or intracranial sepsis due to the dural connection.
Acknowledgments
The authors would like to acknowledge Dr. John Dooley, MBBS, FRCPA from NH Diagnostics for his preparations of the histopathology slides and Prof. Sandeep Bhuta MBBS, FRACR from Department of Radiology, Gold Coast University Hospital for his assistance in interpretation of the radiological images.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://www.theajo.com/article/view/10.21037/ajo-23-9/rc
Funding: None.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://www.theajo.com/article/view/10.21037/ajo-23-9/coif). B.M. serves as an unpaid editorial board member of Australian Journal of Otolaryngology from January 2022 to December 2023. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the Helsinki Declaration (as revised in 2013). The study was approved by the Gold Coast Health and Hospital Service Human Research Ethics Committee (LNR/2022/QGC/70168). Due to the low-risk nature of the reporting, the local research and ethics committee waived the need for informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Quatre R, Baguant A, Gil H, et al. Glial heterotopia of the middle ear. Eur Ann Otorhinolaryngol Head Neck Dis 2020;137:207-9. [Crossref] [PubMed]
- Penner CR, Thompson L. Nasal glial heterotopia: a clinicopathologic and immunophenotypic analysis of 10 cases with a review of the literature. Ann Diagn Pathol 2003;7:354-9. [Crossref] [PubMed]
- Lee DK, Kim JH, Cho YS, et al. Salivary gland choristoma of the middle ear in an infant: a case report. Int J Pediatr Otorhinolaryngol 2006;70:167-70. [Crossref] [PubMed]
- Shim HJ, Kang YK, An YH, et al. Neuroglial Choristoma of the Middle Ear with Massive Tympanosclerosis: A Case Report and Literature Review. J Audiol Otol 2016;20:179-82. [Crossref] [PubMed]
- McGregor DH, Cherian R, Kepes JJ, et al. Case reports: heterotopic brain tissue of middle ear associated with cholesteatoma. Am J Med Sci 1994;308:180-3. [Crossref] [PubMed]
- Lee JI, Kim KK, Park YK, et al. Glial choristoma in the middle ear and mastoid bone: a case report. J Korean Med Sci 2004;19:155-8. [Crossref] [PubMed]
- Farneti P, Balbi M, Foschini MP. Neuroglial choristoma of the middle ear. Acta Otorhinolaryngol Ital 2007;27:94-7. [PubMed]
- Uğuz MZ, Arslanoğlu S, Terzi S, et al. Glial heterotopia of the middle ear. J Laryngol Otol 2007;121:e4. [Crossref] [PubMed]
- Aubry K, Wassef M, Guichard JP, et al. Association of an arachnoid cyst with heterotopic neuroglial tissue in the internal auditory canal]. Ann Otolaryngol Chir Cervicofac 2009;126:133-7. [Crossref] [PubMed]
- Martinez-Peñuela A, Quer S, Beloqui R, et al. Glial choristoma of the middle ear: report of 2 cases. Otol Neurotol 2011;32:e26-7. [Crossref] [PubMed]
- Shemanski KA, Voth SE, Patitucci LB, et al. Glial choristoma of the middle ear. Ear Nose Throat J 2013;92:555-7. [Crossref] [PubMed]
- Wu L, Sun J, Zhang F. Glial heterotopia of the middle ear and Eustachian tube in children. Otolaryngol Head Neck Surg 2013;148:884-5. [Crossref] [PubMed]
- Rizk HG, Lorenz MB, Friedman R. Neuroglial heterotopia of the internal auditory canal. Otol Neurotol 2013;34:e22-3. [Crossref] [PubMed]
- Dunham E, Armeni M. Glial choristoma of the temporal bone in a 7-month-old infant. JAMA Otolaryngol Head Neck Surg 2013;139:944-6. [Crossref] [PubMed]
- Aghaghazvini L, Sharifian H, Rasuli B, et al. Infratemporal Fossa Glial Choristoma (Heterotopia): A Rare Presentation. J Belg Soc Radiol 2016;100:56. [Crossref] [PubMed]
- AlZubaidi Y, Abdulsattar J, Herrera G, et al. True Neuronal Heterotopia of the Middle Ear: A Case Report. Am J Clin Pathol 2018;149:S60-1. [Crossref]
- Pöhlmann Y, Tek F, Verse T. Glial Choristoma in the Middle Ear, A Case Report. Laryngorhinootologie 2021;100:S245.
- Aanaes K, Hasselby JP, Bilde A, et al. Heterotopic neuroglial tissue: two cases involving the tongue and the buccal region. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008;105:e22-9. [Crossref] [PubMed]
- Wolbach SB. Congenital Rhabdomyoma of the Heart. J Med Res 1907;16:495-520.7.
- Heffner DK. Brain in the middle ear or nasal cavity: heterotopia or encephalocele?. Ann Diagn Pathol 2004;8:252-7. [Crossref] [PubMed]
- Ide F, Shimoyama T, Horie N. Glial choristoma in the oral cavity: histopathologic and immunohistochemical features. J Oral Pathol Med 1997;26:147-50. [Crossref] [PubMed]
- Gyure KA, Thompson LD, Morrison AL. A clinicopathological study of 15 patients with neuroglial heterotopias and encephaloceles of the middle ear and mastoid region. Laryngoscope 2000;110:1731-5. [Crossref] [PubMed]
- Glavis-Bloom J, Nahl D, Rubin EM, et al. Congenital neuroglial choristoma of the foot. Radiol Case Rep 2019;14:718-22. [Crossref] [PubMed]
- Şal O, Özen MA, Peker Ö, et al. Two-year old girl with glial choristoma presented in a thyroglossal duct cyst. Turk J Pediatr 2020;62:677-80. [Crossref] [PubMed]
- Plontke SK, Preyer S, Pressler H, et al. Glial lesion of the infratemporal fossa presenting as a soft tissue middle ear mass - rudimentary encephalocele or neural crest remnant?. Int J Pediatr Otorhinolaryngol 2000;56:141-7. [Crossref] [PubMed]
Cite this article as: Hui N, McMonagle B. Glial choristoma of the temporal bone: a case series and literature review. Aust J Otolaryngol 2024;7:7.


