A randomised crossover trial examining the perceived clinical benefits of fenestrated tracheostomy tubes in head and neck patients
Introduction
A tracheostomy is used to facilitate mechanical ventilation, assist secretion clearance, and protect the lungs from gross aspiration of secretions. A tracheostomy also provides or maintains a patent airway, which is particularly relevant in head and neck surgery due to obstruction imposed by post-surgical oedema or as a result of the surgical restoration or repair (1-3).
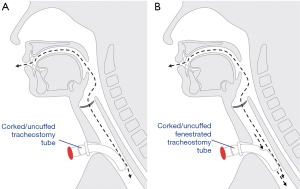
Fenestrated tracheostomy tubes have a hole in the outer aspect of the tube to allow airflow through the upper airway when the fenestration is open. Fenestrated tubes may enable phonation, reduce work of breathing, and assist in the decannulation pathway (4,5). The added airflow may be particularly useful for those undergoing tube occlusion (i.e., corking) prior to decannulation, to compensate for the resistance imposed by the tube itself (6).
Theoretically, fenestrated tubes are advantageous, allowing air to pass both around and through the tracheostomy rather than just around the tracheostomy (Figure 1). In a head and neck surgical population where swelling and anatomical abnormality is common, extra airflow through the upper airway may be important in facilitating speech for patients, as well as improving corking tolerance and subsequent progression towards decannulation.
Clinically however, use of fenestrated tubes is variable because of the perceived risk of harm from fenestration malposition against the tracheal wall, which can result in granulation and subcutaneous emphysema (7-12).
Evidence for and against fenestrated tubes is limited. A systematic review of speech and safety with fenestrated tubes described an overall weak evidence base (8). Evidence supporting fenestrated tubes primarily comes from bench model studies (13). A few clinical studies reporting on fenestrated tracheostomies are primarily small observational studies on critically ill weaning or chronically ventilated patients via a cuffed tube (10,14,15). Tracheostomy-related complications have been reported to be to be seven times more likely with fenestrated versus non-fenestrated tubes, with a subsequent longer wean (12). To our knowledge, there are no prospective clinical studies that provide evidence on the clinical utility of using fenestrated uncuffed tubes as part of a decannulation protocol.
Objectives
The aim of the current study is to determine whether the fenestration does improve airflow in the upper airway, in a head and neck surgical population who have been changed to an uncuffed tube and corked prior to decannulation. Additional aims are to determine if using the fenestration increases airflow available for voicing. We present this article in accordance with the CONSORT reporting checklist (available at https://www.theajo.com/article/view/10.21037/ajo-23-15/rc).
Methods
Ethical considerations
This study was performed in agreement with the ethical principles of the Declaration of Helsinki (as revised in 2013), ICH GCP Notes for Guidance on Good Clinical Practice and the NHMRC National Statement on Ethical Conduct in Research Involving Humans. Approval was obtained from the Royal Adelaide Hospital Human Research Ethics Committee prior to commencement (R20190117) and logged with the Australia and New Zealand Clinical Trials Registry (ACTRN12621001384842). Participants provided informed consent.
Study design
The study design was a randomised, non-blinded, crossover comparison of inner cannulas (fenestrated versus non-fenestrated) within an uncuffed fenestrated outer cannula, to measure primary and secondary outcomes. Patients were their own control; therefore, typical limiting factors in head and neck patient studies such as diagnosis, site and size of lesion, previous radiotherapy, comorbidities such as lung disease and smoking status were minimised by this design.
Setting and participants
The study was conducted in tracheostomised patients under the care of the Ears, Nose & Throat and Oral & Maxillofacial Surgery teams in the Royal Adelaide Hospital, Australia between March 2019 and October 2021. Adult patients (age ≥18 years) who had undergone head and neck surgery, including tracheostomy, were enrolled following their routine postoperative tracheostomy change from a cuffed tracheostomy to an uncuffed fenestrated tracheostomy tube, and once tolerating prolonged occlusion via corking.
Exclusion criteria included:
- Those unable to achieve a lip seal around a spirometer mouthpiece;
- Moribund patients, not expected to survive the hospital episode;
- Patients with language or cognitive barriers unable to perform functional airflow assessment.
Methods and materials
All patients had a Size 6 Shiley CFN Tracheostomy Tube Cuffless with Inner Cannula (Fenestrated) in-situ, with inner diameter 6.4 mm, outer diameter 10.8 mm, and length 76 mm (Medtronic, Sydney, NSW, Australia). The Shiley CFN comes with both fenestrated and non-fenestrated inner cannulas.
Following enrolment (by investigator L.P.), patients were randomised by computer-generated random sequence, in sequentially numbered sealed envelopes, to the order of inner cannula insertion: starting with either fenestrated inner cannula followed by non-fenestrated inner cannula (i.e., order AB), or non-fenestrated inner cannula followed by fenestrated inner cannula (i.e., order BA). Through necessity the tracheostomy was momentarily uncorked during each inner cannula change. Investigators were not blinded to the inner cannula in situ, as the fenestrated cannula was green and the non-fenestrated cannula was white.
Each patient underwent four assessments on a single day, whilst corked, conducted by a single investigator (i.e., order ABAB or BABA), allowing two assessment opportunities with each inner cannula in place (and two paired comparisons for analysis). Assessments took place 60 minutes following each cannula change. These timepoints were chosen to allow multiple opportunities for data collection within a limited timeframe. In the participating hospital, the preferred decannulation protocol is to remove the tube 24 hours following successful corking of a fenestrated tracheostomy, with fenestrated inner cannula in situ.
Main outcome measures
Primary outcome measures of airflow were peak expiratory flow (PEF, L/min) and forced expiratory volume in 1 second (FEV1, L) obtained through incentive spirometry (EasyOne Spirometer, Niche Medical, Perth, WA, Australia) with the best of three samples used for analysis. Where possible the patient’s nose was blocked to minimise air escape, and the patient was sitting upright for assessment to minimise positional impact on airflow measures. The secondary outcome of maximum phonation time (MPT, seconds) was recorded on a digital voice recorder (Voice Memo iPhone application), with the best of three samples used for analysis.
The base plate of the tracheostomy was noted to be flush to the skin and/or tracheostomy dressing prior to each assessment. The fenestration position was observed via tracheoscopy at the first inner cannula change, with the position noted as (I) no obstruction; (II) partial obstruction of fenestration against posterior tracheal wall; and (III) complete obstruction of fenestration against posterior tracheal wall. Patients with obstructed fenestration were not excluded. This was a pragmatic study observing the realities of clinical practice, which did not include fenestration checks and subsequent tube changes if malpositioned.
Analysis
Sample size determination was performed for a paired study design (Wilcoxon non-parametric test) to detect a difference in PEF of 15 L/min with standard deviation (SD) =30 L/min (i.e., effect size =0.5). Assuming alpha =0.05 and power (beta) =0.80, this yielded a total number of patients (n) =28 (i.e., 14 patients for each group). The sample size calculation was done using the G*Power program version 3.1.5 (Universität Düsseldorf, Düsseldorf, Germany).
Linear mixed models were used for analysing the outcomes data, assigning the participant variable as a random effect. The main variable of interest (fenestration) as well as other potential confounding variables such as gender, position of the fenestration against the tracheal wall, and the randomised order of inner canula insertion (either “ABAB” or “BABA”) were analysed as fixed effects. Due to the hierarchical nature of mixed models, in cases of missing data, data from other rows from the same patients were utilised in building these models.
All statistical analyses were performed on R (R Foundation for Statistical Computing, Vienna, Austria) via the Jupyter Notebook frontend.
Results
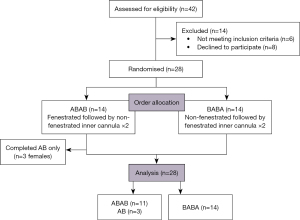
A total of 28 participants were enrolled in the study (Figure 2) with demographics detailed in Table 1. Three of the 28 participants (all female) could not subjectively tolerate repeat testing, and thus had missing data for the 3rd and 4th readings (i.e., data were obtained for AB or BA only).
Table 1
| Parameters | Total cohort (n=28) | Treatment order ABAB (n=14) | Treatment order BABA (n=14) |
|---|---|---|---|
| Age (years) | |||
| Total | 60 [18.6] | 59 [18.7] | 59 [18] |
| Male | 60 [18.6] | ||
| Female | 55 [18] | ||
| Gender | |||
| Male | 20 (71.4) | 9 | 11 |
| Female | 8 (28.6) | 5 | 3 |
| Height (cm) | |||
| Total | 172 [14] | 172 [12.3] | 172 [16] |
| Male | 174 [14] | ||
| Female | 162 [13] | ||
| BMI (kg/m2) | |||
| Total | 24.3 [7.8] | 24.1 [8] | 26 [7.7] |
| Male | 24.3 [7.8] | ||
| Female | 24.3 [7.7] | ||
| Site of lesion/surgery | |||
| Oral tongue | 6 | 3 | 3 |
| Floor of mouth | 4 | 2 | 2 |
| Base of tongue | 2 | 1 | 1 |
| Tonsil | 1 | 0 | 1 |
| Retromolar trigone | 2 | 1 | 1 |
| Mandible | 7 | 4 | 3 |
| Maxilla | 1 | 0 | 1 |
| Supraglottic | 1 | 0 | 1 |
| Pharynx | 1 | 1 | 0 |
| Airway/other | 3 | 2 | 1 |
| Reason for surgery | |||
| Squamous cell carcinoma | 22 | 10 | 12 |
| Osteoradionecrosis | 3 | 2 | 1 |
| Epiglottitis | 1 | 0 | 1 |
| Bilateral vocal cord palsy | 1 | 1 | 0 |
| Ameloblastoma | 1 | 1 | 0 |
| Smoking status | |||
| Never smoked | 4 | 3 | 1 |
| Former smoker | 17 | 7 | 10 |
| Current smoker | 7 | 4 | 3 |
| Days to uncuffed fenestrated tracheostomy post-surgery | |||
| Total | 6 [1.3] | 6 [1] | 6 [1] |
| Days to corking post-surgery | |||
| Total | 7 [5] | 7 [6] | 6 [4] |
| Days to decannulation post-surgery | |||
| Total | 8.5 [7.5] | 9 [8] | 8 [6.5] |
Values are expressed as median [interquartile range], number (%), or number. BMI, body mass index.
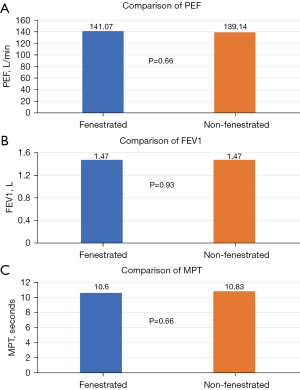
After controlling for gender and the (randomised) order of inner cannula insertion, there was no significant effect of the tracheostomy tube fenestration on PEF (P=0.66), FEV1 (P=0.93), or MPT (P=0.66) (Figure 3). Male gender was significantly associated with a predicted increase of PEF and MPT of approximately 67.7 L/min (P=0.02) and 6.1 seconds (P=0.05), respectively.
Fenestration malposition was observed in 20 of the 28 participants (71%) with half noted to have partial obstruction of the fenestration by the tracheal wall (n=10) and half demonstrating complete obstruction (n=10) (Figure 4). There was higher incidence of malposition in males compared with females (Table 2).
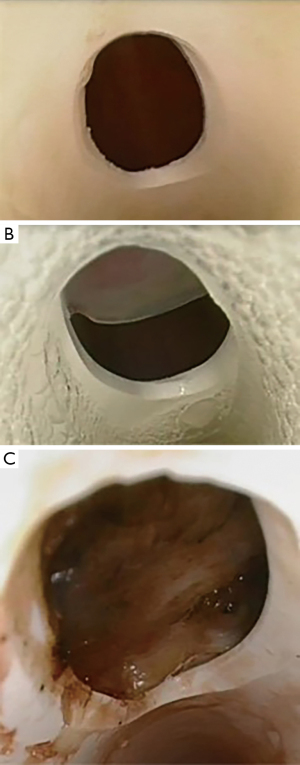
Table 2
| Gender | N (%) |
|---|---|
| Male (n=20) | |
| Fenestration in free space | 2 (10.0) |
| Partial obstruction | 9 (45.0) |
| Complete obstruction | 9 (45.0) |
| Female (n=8) | |
| Fenestration in free space | 6 (75.0) |
| Partial obstruction | 1 (12.5) |
| Complete obstruction | 1 (12.5) |
Analysis of airflow according to fenestration position (no obstruction, partial obstruction, and complete obstruction) was performed. There was no significant difference in PEF, FEV1, or MPT between the three groups in univariable models and in multivariable models (controlling for gender) (Appendix 1).
Subgroup analysis was performed, excluding the ten participants who had their fenestration completely obstructed against the tracheal wall. The multivariable mixed models for this subgroup analysis confirmed again no significant effect of the fenestration but demonstrated significantly higher PEF and MPT in males (Table 3).
Table 3
| Airflow measures | Male | Female | P value |
|---|---|---|---|
| PEF (L/min) | 158.52 | 86.88 | 0.02 |
| Fenestration in free space | 150.01 | 94.62 | |
| Partial obstruction | 164.95 | 117.00 | |
| Complete obstruction | 154.56 | 74.40 | |
| FEV1 (L) | 1.59 | 1.17 | 0.22 |
| Fenestration in free space | 1.74 | 1.35 | |
| Partial obstruction | 1.64 | 0.84 | |
| Complete obstruction | 1.50 | 0.66 | |
| MPT (seconds) | 12.69 | 5.62 | 0.05 |
| Fenestration in free space | 17.38 | 7.11 | |
| Partial obstruction | 11.64 | 3.50 | |
| Complete obstruction | 12.71 | 1.00 |
PEF, peak expiratory flow; FEV1, forced expiratory volume in 1 second; MPT, maximum phonation time.
Three of the eight females reported breathing discomfort when changed from the fenestrated inner cannula to the non-fenestrated inner cannula. These subjective reports were coupled with a small objective change from baseline in oxygen saturations (reduction of >5%) and heart rate (increase of >10%). These participants chose not to complete the crossover testing.
Discussion
The key finding from our study is that fenestration does not increase airflow through the upper airway. When coupled with a high incidence of malposition (71%), particularly in males (90%), our data would not support the routine use of fenestrated tracheostomy tubes in the head and neck cancer post-operative male population.
The fenestration was partially or completely obstructed by the tracheal wall in over two-thirds of the cohort, which could explain the lack of difference in airflow. It has been suggested that “fenestrated tubes often fit poorly and thus do not work as intended” (4) and our findings offer support to this view. However, even in those with the fenestration sitting in free space there were no significant differences noted in airflow.
There were no statistically significant differences according to gender between fenestration and non-fenestration in this study; though, consistent with other reported head and neck caseloads in this region (16,17) females were proportionally less represented than males. However, in the smaller female cohort, over a third did not complete the crossover study protocol due to reduced tolerance of the non-fenestrated inner cannula. The smaller diameter of the female trachea compared to male may explain this observation (18). Corking of a tracheostomy requires the patient to breathe around an obstruction in their airway and as such in a smaller trachea this obstruction is physiologically greater.
Our data support these anatomical differences, with increased measures of PEF and MPT with males compared with females. Trachea size may also impact the fit of the tracheostomy tube, with 90% of males noting malposition compared with only 25% of females. This is not entirely unexpected, given that a size 6 tube is not designed for the dimensions of the male trachea, despite its frequent clinical use in downsizing towards decannulation.
In females, with smaller tracheas, the fenestration is more likely to sit correctly in a position that in turn may be useful to reduce the resistance imposed by the corked tracheostomy tube. Alternatively, another option to fenestrated tracheostomy tubes in females could be the consideration of downsizing to an even smaller diameter uncuffed non-fenestrated tracheostomy tube (e.g., a size 4) to increase the space available around the occluded tube.
It should be noted that published weaning and decannulation protocols consider a change in respiratory observations of greater than 15% from baseline to be clinically significant (19). In our study, the participants who did not complete the full crossover testing showed a change from their baseline observations of less than 15%. The three participants were all allocated to the fenestrated cannula first, followed by change to the non-fenestrated inner cannula (insertion order AB). The cannula changes were non-blinded and therefore participants were aware of the cannula change and a theoretical reduction in space available for airflow. As such, patient anxiety cannot be excluded as a contributing factor to the tolerance of the non-fenestrated inner cannula.
Other potential confounders include the patients’ body mass index (BMI) and impact on respiratory effort (20) with the relationship of the tracheal diameter and BMI an area for further investigation.
The high rate of malposition observed in our study has not previously been reported. In patients who need a longer-term tracheostomy, the impact of the fenestration positioned against the posterior tracheal wall could become problematic. In one retrospective study, nine of 15 patients with a fenestrated tracheostomy developed complications (namely granulation > tracheal stenosis > tracheomalacia) (5). In that research, numbers were very small in the broader context (i.e., only 15 fenestrated tracheostomies from 2,000 tracheostomised patients), but the complication rates were high within this sub-set. Similarly, a retrospective review of 137 weaning tracheostomised patients with fenestrated (n=45) and non-fenestrated (n=89) tracheostomy tubes in situ found that airway complications were significantly higher in those with fenestrated tubes in situ (56% fenestrated compared to 16% non-fenestrated) including greater incidence of granuloma, tube obstruction, and ‘stuck’ tracheostomy tube; with weaning durations longer in the fenestrated group (12).
Overall, our measures of airflow were consistent with but marginally lower than those recently reported for tracheostomised and corked head and neck patients prior to decannulation (6). A leak of air from the stoma cannot be excluded, as the tube in situ for testing was a smaller diameter than the insertional tracheostomy. However, a gauze dressing underneath the tracheostomy flange was in place where possible to minimise any leak, and the crossover design of the study within the same participant ensured the only changing variable was the fenestration.
The MPT in our study was consistent with MPT reported in patients undergoing treatment for head and neck cancer (21).
Comparisons to other studies
To our knowledge, this is the first study to prospectively and comparatively assess the impact of fenestration on airflow and voicing in patients with an uncuffed tracheostomy that has been corked prior to decannulation. Previous research is limited to bench model investigations (13), case studies (10,11), retrospective reviews (5), and in critically ill weaning populations requiring a cuffed fenestrated tube (8,12).
Limitations and strengths
A strength of this study was the fact that patients were their own control, and therefore other variables were minimised. However, we acknowledge the limitations of our research, a key one being that we did not exclude patients for whom the position of the fenestration was observed to be malpositioned. In the conception of this study, we adopted a pragmatic approach for external validity, considering that in a clinical context it had not been standard practice to check the fenestration position and replace each tube if malposition were identified. The study has been driven by a clinical query as to whether or not fenestrated tracheostomy tubes were beneficial as a bridge to decannulation. We did not expect such high incidence of malposition, and this frequency of occurrence has not previously been reported. To have continued recruitment indefinitely to obtain a cleaner sample for analysis did not seem appropriate for our unit, where our current experience tells us that males are the majority cohort, and 90% of males experience malposition.
As the tubes were only in place for a short time, we were unable to report on complications, or show impact on outcomes such as decannulation or length of stay.
Whilst in keeping with other head and neck research, our gender distribution was skewed which may be important, as while not statistically significant there did appear to be gender differences impacting the position and utilisation of the fenestration. It is a reminder that females should always be considered for smaller size endotracheal and tracheostomy tubes than their male counterparts. Our population (male and female) was also predominantly Caucasian. Further investigation in females is warranted, as well as in ethnic male populations where relative trachea size is smaller (22).
Our findings do support the need to visually confirm the position of the fenestration if using these tubes in the future.
Conclusions
In a head and neck surgical population, size 6 fenestrated uncuffed tracheostomy tubes were partially or completely obstructed 71% of the time. Nearly all males (90%) experienced malposition. Fenestrated tracheostomy tubes showed no significant effect in improving three airflow outcomes when corked (FEV1, PEF, MPT). We conclude fenestrated tubes provide no advantage prior to decannulation, in males. In females with small tracheal size, fenestrated tracheostomy tubes may still offer clinical benefit.
Acknowledgments
The authors acknowledge support from Dr. Mary White and Dr. Mark Finnis both from the Royal Adelaide Hospital Intensive Care Unit for their advice regarding study aims and protocol, and Simone Dafoe and Dr. Nathan Ward both from the Royal Adelaide Hospital Physiotherapy Department for their technical advice and equipment provision.
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://www.theajo.com/article/view/10.21037/ajo-23-15/rc
Data Sharing Statement: Available at https://www.theajo.com/article/view/10.21037/ajo-23-15/dss
Peer Review File: Available at https://www.theajo.com/article/view/10.21037/ajo-23-15/prf
Funding: This work was supported by grants from
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure forms (available at https://www.theajo.com/article/view/10.21037/ajo-23-15/coif). L.P. reported that she has received $16,000 in total from grant funding bodies to conduct the research, and conference registration to present the findings from the current manuscript was funded by the South Australian Foundation of Otorhinolaryngology Head & Neck Surgery. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), ICH GCP Notes for Guidance on Good Clinical Practice and the NHMRC National Statement on Ethical Conduct in Research Involving Humans. Approval was obtained from the Royal Adelaide Hospital Human Research Ethics Committee prior to commencement (R20190117) and logged with the Australia and New Zealand Clinical Trials Registry (ACTRN12621001384842). All tubes were purchased as part of standard clinical care. Participants provided informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cheung NH, Napolitano LM. Tracheostomy: epidemiology, indications, timing, technique, and outcomes. Respir Care 2014;59:895-915; discussion 916-9. [Crossref] [PubMed]
- Frutos-Vivar F, Esteban A, Apezteguía C, et al. Outcome of mechanically ventilated patients who require a tracheostomy. Crit Care Med 2005;33:290-8. [Crossref] [PubMed]
- Mallick A, Bodenham AR. Tracheostomy in critically ill patients. Eur J Anaesthesiol 2010;27:676-82. [Crossref] [PubMed]
- Hess DR, Altobelli NP. Tracheostomy tubes. Respir Care 2014;59:956-71; discussion 971-3. [Crossref] [PubMed]
- Pandian V, Boisen SE, Mathews S, et al. Are Fenestrated Tracheostomy Tubes Still Valuable? Am J Speech Lang Pathol 2019;28:1019-28. [Crossref] [PubMed]
- Sanchez-Guerrero JA, Guerlain J, Cebrià I. Expiratory airflow obstruction due to tracheostomy tube: A spirometric study in 50 patients. Clin Otolaryngol 2020;45:703-9. [Crossref] [PubMed]
- Carron MA, Kim SA, Sawhney R, et al. Airway obstruction by granulation tissue within a fenestrated tracheotomy tube: case report. Ear Nose Throat J 2006;85:54-5. [Crossref] [PubMed]
- Pandian V, Boisen S, Mathews S, et al. Speech and Safety in Tracheostomy Patients Receiving Mechanical Ventilation: A Systematic Review. Am J Crit Care 2019;28:441-50. [Crossref] [PubMed]
- Powell HRF, Hanna-Jumma S, Philpott JM, et al. National survey of fenestrated versus non-fenestrated tracheostomy tube use and the incidence of surgical emphysema in UK adult intensive care units. J Intensive Care Soc 2011;12:25-8. [Crossref]
- Pryor LN, Ward EC, Cornwell PL, et al. Establishing phonation using the Blom® tracheostomy tube system: A report of three cases post cervical spinal cord injury. Speech, Language and Hearing 2016;19:227-37. [Crossref]
- Siddharth P, Mazzarella L. Granuloma associated with fenestrated tracheostomy tubes. Am J Surg 1985;150:279-80. [Crossref] [PubMed]
- Shrivastava DK, Kapre S, Gray R. Weaning Is Facilitated by Use of Non-fenestrated Tracheostomy Tubes in Chronically Ill Tracheostomized SubAcute Care Patient. Chest 2003;124:205S. [Crossref]
- Hussey JD, Bishop MJ. Pressures required to move gas through the native airway in the presence of a fenestrated vs a nonfenestrated tracheostomy tube. Chest 1996;110:494-7. [Crossref] [PubMed]
- Kunduk M, Appel K, Tunc M, et al. Preliminary report of laryngeal phonation during mechanical ventilation via a new cuffed tracheostomy tube. Respir Care 2010;55:1661-70. [PubMed]
- Leder SB, Pauloski BR, Rademaker AW, et al. Verbal communication for the ventilator-dependent patient requiring an inflated tracheotomy tube cuff: A prospective, multicenter study on the Blom tracheotomy tube with speech inner cannula. Head Neck 2013;35:505-10. [Crossref] [PubMed]
- Australian Institute of Health and Welfare. Head and neck cancers in Australia. Cancer series No. 83. 2014. Available online: https://www.aihw.gov.au/reports/cancer/head-neck-cancers-australia/summary
- Kao SST, Marshall-Webb M, Dharmawardana N, et al. Gastrostomy tube insertion outcomes in South Australian head and neck cancer patients. Aust J Otolaryngol 2018;1:21. [Crossref]
- Breatnach E, Abbott GC, Fraser RG. Dimensions of the normal human trachea. AJR Am J Roentgenol 1984;142:903-6. [Crossref] [PubMed]
- Rumbak MJ, Graves AE, Scott MP, et al. Tracheostomy tube occlusion protocol predicts significant tracheal obstruction to air flow in patients requiring prolonged mechanical ventilation. Crit Care Med 1997;25:413-7. [Crossref] [PubMed]
- Wang S, Sun X, Hsia TC, et al. The effects of body mass index on spirometry tests among adults in Xi'an, China. Medicine (Baltimore) 2017;96:e6596. [Crossref] [PubMed]
- Ouyoung LM, Swanson MS, Villegas BC, et al. ABCLOVE: Voice therapy outcomes for patients with head and neck cancer. Head Neck 2016;38:E1810-3. [Crossref] [PubMed]
- Tai A, Corke C, Joynt GM, et al. A comparative study of tracheal diameter in Caucasian and Chinese patients. Anaesth Intensive Care 2016;44:719-23. [Crossref] [PubMed]
Cite this article as: Pryor L, Bassiouni A, Dale O, Goggin R, Badlani J, Cheng A, Foreman A, Hodge JC. A randomised crossover trial examining the perceived clinical benefits of fenestrated tracheostomy tubes in head and neck patients. Aust J Otolaryngol 2024;7:11.