Clinical features of responders to mepolizumab in eosinophilic chronic rhinosinusitis and the outcomes post treatment cessation
Introduction
Eosinophilic chronic rhinosinusitis (eCRS) is driven by Th2 eosinophilic inflammation. The majority of eCRS patients can be managed with standard of care treatment including multimodal therapy with intranasal steroids, sinus irrigations and endoscopic sinus surgery (1,2). There are however a small proportion (10%) of eCRS patients who need further ancillary treatment through the use of systemic therapies (3). Biologics have emerged as an adjuvant therapy in the management of eCRS, targeting the different inflammatory pathways driving disease (4).
Mepolizumab is a humanised monoclonal antibody (IgG1, kappa) which targets human interleukin-5 (IL-5) with high affinity and specificity. It has been shown to be effective in the management of eosinophilic asthma, and subsequently guidelines have been developed for the use of biologics in severe asthma (5,6).
There have been limited studies assessing responders to mepolizumab in CRS. An initial study from Bachert et al. [2017] (7) showed reduction in need for surgery and improvement in visual analogue scores (VAS) compared to placebo after 25 weeks. A second study by Gevaert et al. [2011] (8) showed no significant changes in CRS symptom scores at 8 weeks but demonstrated an improvement in total polyp score. More recently, Han et al. [2021] (9) has shown that mepolizumab improves endoscopic nasal polyp score, nasal obstruction VAS and reduces the need for surgery in chronic rhinosinusitis with nasal polyposis (CRSwNP) patients.
Indications for treatment of CRSwNP with biologics have been extensively discussed amongst rhinologists (2,10). These include evidence of type 2 inflammation, need for systemic steroids, impairment of quality of life, loss of smell and the presence of co-morbid asthma (10). However, defining the response to treatment can be challenging due to the substantial variability in response amongst patients.
As mepolizumab has now been subsidised by the Pharmaceutical Benefits Scheme (PBS) as of April 2023, identifying patients who are treatment responders compared to non-responders is of key importance to both defining and optimising the role of biologics in the management pathway of eCRS. The outcomes of CRS patients following cessation of mepolizumab therapy are yet to be assessed. The aim of this study is to review the clinical outcomes of eCRS patients during treatment and following cessation of mepolizumab.
Methods
A prospective open-label single-arm single-centre study of non-blinded patients undergoing mepolizumab treatment through a phase 2 clinical trial at a tertiary ENT practice was performed (trial registration ID ACTRN12618000113257). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study received ethics approval from the St Vincent’s Hospital Human Research Ethics Committee (REGIS ID 2019/PID04424) and participants provided written informed consent for data collection. The goal of the study was to define the clinical and disease features of those patients who reported benefit from therapy compared to non-responders.
Adult patients (≥18 years) diagnosed with eCRS [based on sinonasal tissue eosinophilia >10 per high power field (HPF) (400× magnification) on at least two HPFs, on a biopsy during which all patients had ceased systemic corticosteroid medications at least 4 weeks beforehand] (11) and assessed by a tertiary rhinologist as having disease not controlled by the current standard of care, requiring biologic therapy for management of their disease (3). Exclusion criteria included previous treatment with mepolizumab, known hypersensitivity to mepolizumab, known immunodeficiency, cystic fibrosis, pregnancy, or current lactation. The study design is summarised in Table 1.
Table 1
| Interventions | Visit 1 (baseline) | Visit 2 | Visit 3 | Visit 4 | Visit 5 | Visit 6 (last dose) |
Visit 7 (end-of-treatment) |
Visit 8 (post-treatment) |
|---|---|---|---|---|---|---|---|---|
| Time (weeks) | 0 | 4 | 8 | 12 | 16 | 20 | 24 | 36 |
| Blood eosinophil | √ | √ | √ | √ | √ | √ | √ | √ |
| Tissue eosinophil | √ | √ | √ | √ | √ | |||
| Questionnaires: SNOT-22, ACQ-5 | √ | √ | √ | √ | √ | √ | √ | √ |
| FeNO, nNO | √ | √ | √ | √ | √ | √ | √ | √ |
| Endoscopy | √ | √ | √ | √ | √ | √ | √ | √ |
| Spirometry | √ | √ | √ | √ | √ | √ | √ | √ |
| Adverse event assessment | √ | √ | √ | √ | √ | √ | √ | √ |
| Administration of mepolizumab | √ | √ | √ | √ | √ | √ | ||
| Overall treatment response | √ | √ |
nNO, nasal nitric oxide; SNOT-22, Sino-Nasal Outcome Test 22-item; ACQ-5, Asthma Control Questionnaire 5-item; FeNO, fractional exhaled nitric oxide.
Biologic therapy
Patients received mepolizumab 100 mg via a subcutaneous injection once every 4 weeks for a total of 20 weeks for a total of 6 doses. Data was collected at baseline and at 4, 8, 12, 16, 20 and 24 weeks of treatment (‘end of treatment’). A further review was performed 4 months following the last dose of mepolizumab (‘post treatment’) at 36 weeks.
Demographic data
Demographic characteristics were recorded, including age, gender, asthma status, atopy, smoking status, date of previous surgery, and use of intranasal and systemic corticosteroid medication. Asthma status was determined by either a 15 percent change in forced expiratory volume in 1 second (FEV1) on spirometry with challenge testing or b-agonist use, or current use of regular inhaled bronchodilator or corticosteroid therapy.
Atopy was defined as ≥1 positive result in either skin prick allergy testing (SPT) or automated immunoassay (ImmunoCap®) to detect serum-specific immunoglobulin (Ig) E antibodies to the following 4 aeroallergen mixes: (I) grass mix; (II) dust mite; (III) mould and (IV) animal epithelium. A serum-specific IgE level of greater than 0.35 kU/L for any of these aeroallergen mixes was considered a positive result and classified as atopic.
Smoking status was defined by having smoked within the last 12 months. Current medications (name and dosage) were recorded at screening and reviewed at each treatment visit. Patients continued their current standard of care: intranasal corticosteroid nasal spray and sinus irrigations as per EPOS 2020. All trial participants were precluded from changing medications in the four weeks prior to commencement of and during mepolizumab treatment. Patients were not permitted to have oral corticosteroid courses for the duration of the study.
Blood eosinophil count
At each visit, a blood sample was taken to assess for total blood eosinophil count (cells ×109/L), measured by automated analysis (Haematology Analyzer, DxH 800, Beckman Coulter, Brea, USA).
Tissue eosinophil count
Sinonasal tissue biopsies were taken from the sinonasal cavity of representative diseased tissue at weeks 0, 8, 16, 24 and 36. Samples were placed in formalin, processed with standard hematoxylin and eosin (H&E) staining and assessed by pathologists blinded to the clinical data. Eosinophil count per square millimetre and degree of inflammation was evaluated.
Spirometry
Spirometry was performed using a closed-circuit method. FEV1 and forced vital capacity (FVC) was measured as both total volume in litres as well as percentage of predicted. Due to the SARS-CoV-2 pandemic, spirometry was not performed during the period of April to August 2020 due to the risk of aerosolization of particles.
Rhinomanometry
Total nasal airway resistance (NAR) was measured at each visit by four-phase active anterior rhinomanometry using an NR6 Rhinomanometer (GM Instruments, UK), following the international standard of 150 Pa. One nostril was occluded with a foam plug attached to the pressure sensor, and secured with micropore tape. An anaesthetic-styled mask was positioned and held by the patient against the face, covering from the bridge of the nose to the chin to ensure an airtight seal. The patient was then instructed to perform tidal breathing through the patent nostril with the mouth closed. The test was repeated to achieve reproducibility of 10% within two readings, and the procedure repeated in the contralateral side. One representative reading for each side was subsequently selected and combined using NARIS software (GM Instruments, UK) to determine total NAR (Pa/cm3/s).
Sino-Nasal Outcome Test 22-item (SNOT-22)
The SNOT-22 is a validated disease specific quality of life questionnaire established in CRS and was completed at each trial visit (12). Each component is scored on a 6 point Likert scale of 0 to 5 (0= no problem, 1= very mild problem, 2= slight problem, 3= moderate problem, 4= severe problem, 5= as bad as it can be). A total score out of 110 was compiled based off 22 questions and a higher score indicates more severe symptoms. Independent assessment of the nasal obstruction SNOT-22 component from 0 to 5 was also performed.
SNOT-22 subdomains (rhinologic, extra-rhinologic, ear/facial, psychological and sleep) were assessed (13). Additionally, a nasal symptom score (NSS) was also collected by totaling five components of the SNOT-22 (need to blow nose, thick nasal discharge, facial pain/pressure, loss of smell/taste and nasal obstruction components).
Asthma Control Questionnaire 5-item (ACQ-5)
The ACQ-5 is a validated questionnaire consisting of five questions assessing asthma symptoms and control over the past week and was completed at each trial visit. Each component is scored separately on a Likert scale of 0 to 6 in order of increasing severity, where 0 represented excellent asthma control and 6 represented very poor asthma control. The overall ACQ-5 is calculated as the average of the five questions.
Nitric oxide assessment
Fractional exhaled nitric oxide (FeNO) and nasal nitric oxide (nNO) were measured with a NIOX VERO® analyser (Aerocrine AB, Stockholm, Sweden) at each visit, using a closed circuit technique in accordance with the American Thoracic Society guidelines (14). FeNO measurement was measured through initial inhalation to total lung capacity (TLC) through a filter allowing for elimination of background exhaled NO. This was followed by slow oral exhalation at a constant 50 mL/s flow rate for at least 10 seconds where FeNO was measured once reaching steady-state plateau (15). NO was recorded using the expiration against resistance method with a nasal olive sensor placed in the dominant nare with a tight seal to avoid ambient air sampling. Following initial inhalation of room air to TLC, patients exhaled orally against a restrictor for 30 seconds, generating a pressure which results in soft palate closure. With this now closed-circuit nasal cavity, air is then aspirated for 30 seconds via the nasal sensor and analysed by the machine (16).
Nasal endoscopy
Endoscopic appearances of the sinonasal cavity were recorded at each visit and assessed by two blinded reviewers. This was assessed with a Modified Lund Mackay Postoperative Endoscopy Score (MLMES), assessing changes in oedema, purulence and discharge in each of the maxillary, ethmoid, sphenoid, frontal sinuses and olfactory fossa (17). Total MLMES, oedema, purulence and discharge scores were scored.
Medication safety
Medication safety was evaluated using continuous adverse events reporting at each clinical visit. Known adverse reactions to mepolizumab include headache, injection site reaction, back pain, fatigue, nasopharyngitis (18,19). Described manifestations of systemic hypersensitivity reactions included rash, pruritis, headache and myalgias (20).
Responders to therapy
An overall nasal function score was measured on a visual analogue scale (VAS) with a range between −6 and +6. Responders to therapy were defined as those having an improvement in overall nasal function VAS of ≥1 post-treatment. This is similar to previous studies assessing biologics in eCRS where VAS score related to nasal function was recorded (e.g., nasal obstruction score, nasal congestion/obstruction score) (9,21).
Statistical analysis
The primary endpoint of the study was assessing outcomes post-treatment (4 months following the last dose of 6 months of mepolizumab treatment) in comparison to end-of-treatment and baseline.
Statistical analysis was conducted using SPSS Statistics version 26 (IBM Corp., Armonk, NY, USA). Paired t-test comparisons were used for parametric continuous variables. Chi square and Fisher’s Exact tests were performed for relationships of nominal variables. All P values were two-tailed, and a value of P<0.05 was considered statistically significant. All parameters were assessed in a blinded fashion.
Results
Twenty patients (age 47.7±14.5 years, 50% female) were recruited from a period of May 2019 to March 2020. They were 4.1±3.5 years since their last surgery. All patients had co-morbid asthma and 50% were atopic. Baseline demographic data is summarised in Table 2. Data was collected from May 2019 to November 2020.
Table 2
| Patient demographics | Value, n=20 |
|---|---|
| Age (years) (mean ± SD) | 47.7±14.5 |
| Gender (% female) | 50 |
| Years since last surgery (mean ± SD) | 4.1±3.5 |
| Number of previous surgeries | |
| 0 | 0 |
| ≥1 | 3 |
| ≥2 | 5 |
| ≥3 | 5 |
| ≥4 | 3 |
| ≥5 | 4 |
| Asthma (%) | 100 |
| Atopy (%) | 50 |
| Smoking (%) | 15 |
| Intranasal corticosteroids (%) | 50 |
| Systemic corticosteroids (%) | 10 |
| Treatment response at 6 months, n [%] | 14 [70] |
SD, standard deviation.
Treatment with mepolizumab therapy
Results are summarised in Table 3.
Table 3
| Parameter | Baseline | 6 months | P value |
|---|---|---|---|
| Blood eosinophil (cells ×109/L) | 0.48±0.28 | 0.08±0.07 | <0.0001* |
| Tissue eosinophils (cells/0.1 mm2) | 101.64±93.80 | 41.74±53.76 | 0.035* |
| SNOT-22 (0–110) | 45.1±19.9 | 30.6±22.5 | 0.007* |
| ACQ-5 (0–5) | 1.97±1.1 | 1.0±1.0 | 0.001* |
| FEV1/FVC (%) | 73.5±7.0 | 74.2±7.0 | 0.43 |
Results are expressed as mean ± standard deviation. *, indicates significant results. SNOT-22, Sino-Nasal Outcome Test 22-Item; ACQ-5, Asthma Control Questionnaire 5-item; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity.
Overall nasal function
Overall nasal function VAS significantly improved (−2.5±3.4 vs. 1.5±3.3, P<0.001) as well as nasal symptom score (14.5±5.2 vs. 9.5±5.9, P=0.002) from baseline to end-of-treatment.
Blood eosinophil count
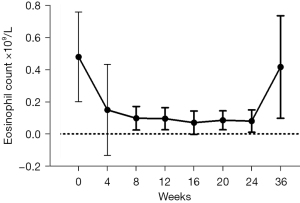
There was a significant decrease in blood eosinophil count following 4 weeks of therapy [(0.48±0.28) vs. (0.1±0.1)×109/L, P=0.001] and sustained through to end-of-treatment [(0.48±0.28) vs. (0.08±0.07)×109/L, P<0.0001] (Figure 1).
Tissue eosinophil count
There was a significant decrease in tissue eosinophil count from baseline to end-of-treatment (101.64±93.80 vs. 41.74±53.76 cells/0.1 mm2, P=0.035).
SNOT-22
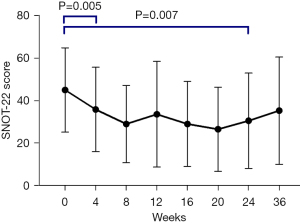
Early improvement in SNOT-22 was evident from baseline compared with 4 weeks (45.1±19.9 vs. 35.9±20.0, P=0.005). This improvement from baseline continued to end-of-treatment (45.1±19.9 vs. 28.8±21.7, P=0.007) (Figure 2).
Significant improvements were shown in the rhinologic, extra-nasal rhinologic and psychologic subdomains, however, there were no significant changes in the ear/facial or sleep subdomains (Table S1).
ACQ-5
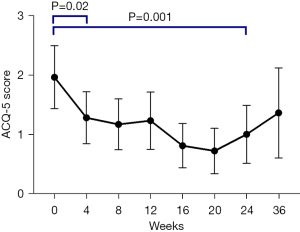
Patients’ asthma control subjectively improved from baseline to 4 weeks with mepolizumab (2.0±1.1 vs. 1.3±0.9, P=0.02). This clinical improvement continued during therapy with significant improvement from baseline to end-of-treatment (2.0±1.1 vs. 1.0±1.0, P=0.001) (Figure 3). The ACQ-5 domains all significantly improved from baseline to end-of-treatment (Table S1).
Spirometry
There were no significant changes in FEV1/FVC from baseline to 6 months. FEV1/FVC (n=10, 73.5%±7.0% vs. 74.2%±7.0%, P=0.43). Interestingly, there was an initial improvement from baseline at the 4-week visit (n=18, 72.9%±8.0% vs. 75.8%±7.2%, P=0.03), however this was not sustained through the course of treatment.
Rhinomanometry
There were no significant differences in rhinomanometry from baseline to end-of-treatment (0.10±0.03 vs. 0.10±0.02, P=0.25).
FeNO
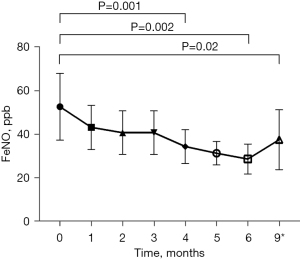
There was a significant decrease in FeNO from commencement to 4 months of treatment (53±33 vs. 34±17 ppb, P=0.001). Compared to baseline, this improvement in FeNO was sustained following total 6 months of treatment (53±33 vs. 29±14 ppb, P=0.002) and continued to the 3-month post-treatment measurement (53±33 vs. 38±28 ppb, P=0.02) (Table S1) (Figure 4). However, whilst there was not a significant difference between end of treatment and 4 months post-treatment (29±14 vs. 38±28 ppb, P=0.06), this was close to the minimal clinically important difference of 20% (21).
nNO
There were no significant changes in nNO from baseline following 6 months of treatment with mepolizumab (404±269 vs. 377±327 ppb, P=0.65). There was no correlation between FeNO and nNO (r=0.15, P=0.06). Pearson-r correlation showed non-significant association between peripheral blood eosinophils with FeNO (r=0.20, P=0.01) and nNO (r=−0.20, P=0.01). Pearson-r correlation performed between SNOT-22 and FeNO showed no association between the two variables (r=−0.09, P=0.26) and weak correlation between SNOT-22 and nNO (r=−0.18, P=0.03).
Nasal endoscopy
The MLMES was not significantly different between baseline to end-of-treatment (42.9±11.2 vs. 33.6±22.2, P=0.09). Total oedema score was similar between baseline and end-of-treatment (33.9±8.8 vs. 27.4±18.2, P=0.17) (Table S1).
Responders to mepolizumab therapy
Fourteen patients out of 20 (70%) were assessed to have responded to mepolizumab therapy after 6 months (average improvement in overall nasal function VAS 5.6±3.2). Four patients reported similar overall nasal function VAS to baseline and 2 patients reported worsened overall nasal function VAS.
Comparison of baseline markers between responders and non-responders are shown in Table 4 and Figure 5. There were no significant differences in blood eosinophil count [(0.51±0.30) vs. (0.42±0.24)×109/L, P=0.52] or tissue eosinophil count (108.0±101.8 vs. 74.5±69.5 cells/0.1 mm2, P=0.48) between responders and non-responders. FEV1/FVC was similar between responders and non-responders (73.5%±7.8% vs. 67.6%±9.1%, P=0.16). Responders had lower baseline nNO compared to non-responders (300.4±169.7 vs. 645.0±318.7 ppb, P=0.005) (Figure 5A) but no significant differences in FeNO (52.6±38.5 vs. 52.5±13.8 ppb, P=0.99).
Table 4
| Parameter | Responder (n=14) | Non-responder (n=6) | P value |
|---|---|---|---|
| ∆ Overall nasal function | 5.6±3.2 | −0.8±1.6 | <0.001* |
| Blood eosinophil (cells ×109/L) | 0.51±0.30 | 0.42±0.24 | 0.52 |
| Tissue eosinophil (cells/0.1 mm2) | 108.0±101.8 | 74.5±69.5 | 0.48 |
| FEV1/FVC (%) | 73.5±7.8 | 67.6±9.1 | 0.16 |
| FeNO (ppb) | 52.6±38.5 | 52.5±13.8 | 0.99 |
| nNO (ppb) | 300.4±169.7 | 645.0±318.7 | 0.005* |
Data are presented as mean ± standard deviation. Responders defined as overall nasal function ≥1 point change over 6 months. *, indicates significant results. FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; FeNO, fractional exhaled nitric oxide; nNO, nasal nitric oxide.
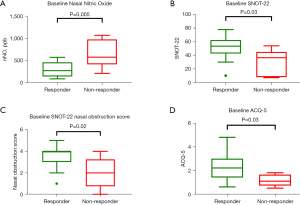
Baseline patient reported outcome measures between responders and non-responders are summarised in Table 5. At baseline, responders had higher SNOT-22 compared to non-responders (51.3±17.3 vs. 30.7±19.1, P=0.03) (Figure 5B). Responders reported poorer baseline SNOT-22 nasal obstruction score in comparison to non-responders (3.5±1.1 vs. 2.0±1.5, P=0.02) (Figure 5C). Whilst the majority of SNOT-22 domains were similar between the two groups, the SNOT-22 psychological subset was significantly worse in responders than non-responders (15.2±7.4 vs. 6.2±5.8, P=0.02).
Table 5
| Parameter | Responder (n=14) | Non-responder (n=6) | P value |
|---|---|---|---|
| SNOT-22 | 51.3±17.3 | 30.7±19.1 | 0.03* |
| SNOT-22 nasal obstruction score | 3.5±1.1 | 2.0±1.5 | 0.02* |
| Psychological subdomain | 15.2±7.4 | 6.2±5.8 | 0.02* |
| SNOT-22 psychological subdomain | 2.3±1.2 | 1.2±0.5 | 0.03* |
| ACQ-5 morning symptoms | 2.5±1.3 | 0.7±1.0 | 0.008* |
| ACQ-5 activities | 2.2±1.7 | 0.7±0.5 | 0.007* |
| Lund-Mackay score | 40.8±8.5 | 43.7±12.4 | 0.61 |
Data are presented as mean ± standard deviation. *, indicates significant results. SNOT-22, Sino-Nasal Outcome Test 22-Item; ACQ-5, Asthma Control Questionnaire 5-item.
Responders also reported significantly worse ACQ-5 scores than non-responders (2.3±1.2 vs. 1.2±0.5, P=0.03), notably in the morning symptoms and limitations of activities domains (Table 5) (Figure 5D). There were no significant differences in baseline endoscopic findings between responders and non-responders (Table 5) (Table S2).
Binomial regression analysis did not identify any significant predictive markers of response to mepolizumab therapy.
Post-mepolizumab treatment outcomes
Eighteen patients were reviewed 4 months following the last dose of mepolizumab therapy (‘post-treatment’). The remaining two patients continued with mepolizumab and were therefore not included. Post-treatment outcomes are summarised in Table 6.
Table 6
| Parameter | End of treatment | Post treatment | P value |
|---|---|---|---|
| Blood eosinophil (cells ×109/L) | 0.07±0.07 | 0.42±0.32 | 0.001* |
| Tissue eosinophil (cells/0.1 mm2) | 41.7±53.8 | 76.3±98.2 | 0.06 |
| SNOT-22 | 28.8±21.7 | 35.3±25.3 | 0.11 |
| ACQ-5 | 0.9±1.0 | 1.3±1.5 | 0.15 |
| FEV1/FVC (%) | 74.2±7.0 | 91.5±9.8 | <0.001* |
| FeNO (ppb) | 27.6±12.7 | 37.9±28.4 | 0.06 |
| nNO (ppb) | 389.7±344.1 | 396.5±231.4 | 0.93 |
Data are presented as mean ± standard deviation. *, indicates significant results. SNOT-22, Sino-Nasal Outcome Test 22-Item; ACQ-5, Asthma Control Questionnaire 5-item; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; FeNO, fractional exhaled nitric oxide; nNO, nasal nitric oxide.
Blood eosinophil count
Following cessation of mepolizumab therapy, post-treatment blood eosinophil count was significantly higher than end-of-treatment values [(0.42±0.32) vs. (0.07±0.07)×109/L, P=0.001]. These results were similar to baseline levels [(0.42±0.32) vs. (0.48±0.30)×109/L, P=0.55].
Tissue eosinophil count
Post-treatment, tissue eosinophils also showed increase count compared to end-of-treatment (76.3±98.2 vs. 41.7±53.8 cells/0.1 mm2, P=0.06) however this was not statistically significant. Post-treatment tissue eosinophil count was similar to baseline levels (76.3±98.2 vs. 97.9±92.8 cells/0.1 mm2, P=0.33).
SNOT-22
SNOT-22 outcomes were not significantly different between post-treatment compared with end-of-treatment (35.3±25.3 vs. 28.8±21.7, P=0.11). Whilst SNOT-22 scores improved post-treatment compared with baseline, this was not statistically significant (35.3±25.3 vs. 44.9±20.2, P=0.09). SNOT-22 nasal obstruction score was not significantly different between post-treatment and end-of-treatment (P=0.51); however post-treatment remained significantly improved compared to baseline (2.1±1.7 vs. 3.1±1.4, P=0.008). CRS patient reported outcome measures are detailed in Table S3.
ACQ-5
Post-treatment ACQ-5 overall worsened but was not significant different compared to end-of-treatment values (1.4±1.3 vs. 1.0±1.0, P=0.08). Post-treatment ACQ-5 was statistically similar to baseline ACQ-5 values (1.4±1.3 vs. 2.0±1.1, P=0.07).
Spirometry
Interestingly, FEV1/FVC significantly improved following cessation of mepolizumab therapy in the post-treatment values compared to end-of-treatment (n=10, 91.5%±9.8% vs. 74.%2±7.0%, P<0.001). Post-treatment FEV1/FVC was also significantly improved compared to baseline (n=18, 84.5%±13.7% vs. 72.5%±8.5%, P<0.001).
Rhinomanometry
There were no significant differences in rhinomanometry post-treatment compared to end-of-treatment (0.09±0.03 vs. 0.10±0.02, P=0.22). There were no significant differences in post-treatment compared with baseline (P=0.19).
nNO
No significant differences were found between post-treatment assessment and end-of-treatment nNO (396.5±231.4 vs. 389.7±344.1 ppb, P=0.93).
Nasal endoscopy
There were no significant differences in MLMES between post-treatment compared with end-of-treatment (36.2±18.4 vs. 35.4±23.0, P=0.82). There were no significant differences between post-treatment and baseline scores (P=0.10). There were no significant differences in post-treatment total oedema score compared with end-of-treatment (P=0.96) or baseline (P=0.07) (Table S3).
Discussion
Evidence is accumulating for the role of biologics as a safe addition to the management of CRSwNP (22). Mepolizumab has been successful in the management of upper airway eosinophilic inflammatory disease since it received FDA approval in November 2015 and recently approved by PBS in April 2023 (23). This study assessed the clinical features of responders and non-responders to mepolizumab, as well as assessing the post-treatment outcomes in eCRS.
Study participants were defined based on both tissue confirmation of eCRS (based on prior sinonasal biopsy, during which all patients had ceased systemic corticosteroid medications at least 4 weeks beforehand) as well as failure of current standard of treatment for CRS (1). Defining eCRS based on tissue histopathology (and confirmed on baseline tissue histopathology) allowed this study to assess the effect of mepolizumab on a group of patients with CRS driven by Th2 dominant inflammation at the sinonasal mucosal level, which had not been previously studied.
Responders to treatment in this study were defined as improvement in the overall nasal function score of at least 1 point. In this study 70% of patients were assessed to have responded to mepolizumab. This was similar to previous studies looking at mepolizumab in CRS which assessed VAS related to nasal symptoms. Han et al. [2021] showed 71% of patients receiving mepolizumab showed ≥1 point improvement in nasal obstruction VAS from baseline compared to those with placebo (9). Three months post cessation of therapy, overall nasal function worsened but this was not significantly worse than end-of-treatment. This suggests a mild enduring effect of treatment at the local tissue level.
In this study, binomial regression analysis did not identify specific predictive markers of response to mepolizumab therapy, likely secondary to the pilot nature of this study. Responders to treatment were more likely to have a higher SNOT-22, ACQ-5 and lower nNO at baseline. nNO expression is influenced by a number of anatomical, pathological and physiological contributors and higher nNO levels are inversely correlated with the extent of sinonasal inflammatory disease. It can be hypothesised that patients with more severe disease burden may observe more significant improvement to therapy and significant improvement in their overall nasal function. This is similar to findings in eosinophilic asthma which demonstrated that super-responders to mepolizumab were more likely to have a T2 disease burden (24).
Notably, responders to therapy did not improve across all outcomes measured in this study. The minimally clinically important difference (MCID) for SNOT-22 has been shown to be 8.9 (25). In this study 8 out of 14 responders (57.1%) achieved at least a MCID in their SNOT-22 following 6 months of treatment, compared to 1 out of 6 non-responders (16.7%). Regarding asthma disease control, 9 out of 14 (64%) responders achieved an MCID in their ACQ-5 of ≥0.5 compared to 3 out of 6 (50%) non-responders (26,27). The MLMES also improved in 64% of responders compared with 50% non-responders.
Whilst limited by a small study population, the significant variability in response across these clinical markers in responders suggest a need for a multifaceted approach to identifying patients most likely to benefit from biologics as well as assessing outcomes in treatment with biologics.
Considering the limited data for an optimal responder population at this current time, a trial of treatment based on current selection criteria is reasonable, however careful analysis to identify non or poor responders at 6 months is warranted. Defining appropriate eligibility criteria for biologic therapy as well as indicators for continuing and/or ceasing therapy will be important factors in effective usage of mepolizumab for eCRS for the future.
Blood eosinophils have been demonstrated as a useful biomarker for response to therapy in CRS and eCRS, reflecting clinical improvement (28-30). Blood eosinophils are also used as a biomarker for the monitoring of treatment response to biologics in eosinophilic asthma. In both eosinophilic asthma and CRSwNP studies, subgroup analysis have shown that the efficacy of mepolizumab was related to higher blood eosinophil count at baseline (9,24). However, this was not demonstrated in this study, potentially due to the smaller patient population.
This study showed a significant decrease in blood eosinophils following the first dose of mepolizumab, and this persisted throughout treatment. Three months after cessation of treatment, post-treatment blood eosinophils significantly increased compared to end-of-treatment, returning to baseline or pre-treatment levels. Similar to tissue eosinophils (31), blood eosinophils appear promptly sensitive to mepolizumab initiation and cessation (Figure 2), which further strengthens blood eosinophils role as a convenient and suitable biomarker in the monitoring of treatment response in eCRS.
Whilst FEV1/FVC initially improved following mepolizumab therapy, this was not sustained at the 8 week and subsequent assessment visits during treatment. It should be noted that given this trial was performed partly during the COVID-19 pandemic, hence spirometry data was not collected for ten patients at the end of treatment visit (week 24) due to concerns of aerosolization of respiratory particles during testing. The small number (n=10) at end of treatment assessment may account for the non-significant results at this time point compared to baseline.
Interestingly, post-treatment, FEV1/FVC ratio was significantly improved in paired comparisons to both baseline and end of treatment visit. This suggests that even in patients with primary upper airways eosinophilic disease, mepolizumab can improve functional lower airways outcomes in patients with comorbid asthma. Studies in eosinophilic asthma have shown modest but clinically significant improvement in FEV1 and FVC compared to baseline (32,33). However this effectiveness has been shown to wane following 24 weeks of treatment, with no clinically significant differences at week 200 in FEV1 in eosinophilic asthma (18). Improvements in eosinophil driven airway inflammation are not thought to have a direct effect on lung function (18).
In this study, the mechanism of action of delayed improvement of FEV1/FVC following end of treatment is unknown, and may be confounded by the limited measurements at end of treatment visit (n=10). Haldar et al. [2014] showed no significant differences in post-bronchodilator FEV1 for 12 months following cessation of mepolizumab (34).
In the studied eCRS population, statistically significant reduction of FeNO levels were achieved following 4 months of treatment with mepolizumab (53±33 vs. 34±17 ppb, P<0.001). There were further significant reduction of FeNO levels from 4 to 6 months of treatment (34±17 vs. 29±14 ppb, P=0.01) which was sustained 4 months post the last dose. This was associated with improvement in asthma control, both based on GINA classification and ACQ-5—suggesting that this may be secondary to improvement in both upper and lower airway inflammation. Whilst blood eosinophils dramatically decrease following the first dose, the findings suggest delayed responsiveness of FeNO levels to mepolizumab only after 4 months. This may be explained by treatment causing a gradual reduction in the overall amount of inflammation within the respiratory epithelium. Whilst no direct mechanisms between IL-5 inhibition and NO have been reported, we hypothesise that this may occur through mepolizumab’s indirect inhibition of the IL-4/IL-13 pathway (35-37). A predictive role for nitric oxide in eosinophilic asthma has been proposed by Couillard et al. [2021] with an Oxford Asthma Attack Risk Scale (ORACLE) to predict asthma attacks using a combination of blood eosinophil count and FeNO (38).
In this study, baseline nNO was significantly lower in CRS responders than in non-responders. There are multiple contributors to decreased levels of nNO including mucociliary dysfunction, obstructive remodelling changes and anatomical variability in eCRS (39). Although the mechanism of action is not entirely clear in this study, impaired sinonasal production of nNO (i.e., low nNO levels) may act as a marker of more severe inflammation, which in turn may be more sensitive to IL-5 inhibition by mepolizumab. The usefulness of nNO as a biomarker in inflammatory sinus disease requires further investigation due to the complex variability in and interaction between the anatomical, pathological and physiological contributors to NO production (40).
Whilst the SYNAPSE study showed significant improvement in total endoscopic nasal polyp score after 12 months (9), in this study endoscopic appearances were not significantly improved following 6 months of treatment. Our methodology assessed endoscopic appearances of the sinonasal cavity using the MLMES, as all patients were post-operative. MLMES assesses all ten sinus cavities (the left and right maxillary, ethmoid, sphenoid and frontal sinuses and olfactory fossa). This differs to the total endoscopic score used in the SYNAPSE study which was scored using the Meltzer clinical scoring system which only analyses polyp appearances related to the middle meatus (41).
The lack of significant improvement in the endoscopic appearances in this study may be explained by patient selection or duration of therapy. SYNAPSE patient selection included a more severe patient group as only patients with endoscopic nasal polyps score of ≥5, with a minimum score of 2 in each nasal cavity were included (9). On the other hand, this study’s eligibility criteria did not include sinonasal appearances and baseline total oedema score ranged from 21 to 51. As endoscopy is a useful way to monitor disease and determine need for operative intervention whilst on biologic treatments in eCRS, additional research is required to guide clinicians regarding continuing or ceasing mepolizumab as well as timing/necessity of revision surgery.
Longer term cessation post treatment outcomes have been reported in eosinophilic asthma studies: Ortega et al. [2019] described 3-month post-treatment outcomes following 12 months of mepolizumab, with worsening of ACQ-5 to a mean of 1.66, as well as a significant elevation in blood eosinophils (42). Haldar et al. [2014] assessed 12-month post treatment outcomes in severe eosinophilic asthma, again showing significant increase in blood eosinophils and clinical symptom worsening (34). These findings in eosinophilic asthma are similar to the 4 month post treatment scores in eCRS found in this study: rise in blood eosinophils, worsening of SNOT-22 and ACQ-5 were noted following cessation of treatment. This data supports treatment efficacy whilst on the drug but no evidence of sustained disease remission.
Defining appropriate eligibility criteria for biologic therapy as well as indicators for continuing and/or ceasing therapy will be important factors in effective usage of mepolizumab for eCRS. This study had a comprehensive approach in assessing local tissue, systemic, endoscopic as well as subjective reported outcomes in response to mepolizumab therapy. No significant adverse outcomes were noted during the course of the study. Local tissue biopsy was associated with minor discomfort and bleeding. However, tissue biopsy allowed for a more extensive understanding of the clinical outcomes.
Due to the pilot nature of the study, it was limited by the small patient population, which therefore limited the modelling and identification of potential biomarkers. No control or placebo arm was able to be included in the design of this study which further limits interpretation. Further studies assessing the long-term outcomes following treatment and cessation of mepolizumab in the eCRS population are required to guide management.
Whilst mepolizumab is clinically effective, markers for (I) continuation, (II) cessation of therapy, and (III) conversion to operative intervention should be clearly defined before wider use. Cost-effectiveness is an important consideration prior to initiation, especially in cases where patients are self-funded. Health economic cost analysis should be considered to further evaluate the role of biologics in the management of CRS and eCRS.
Conclusions
Mepolizumab is effective adjuvant therapy in the management of eCRS, with associated clinical deterioration following cessation of therapy. Further studies are required to determine a population most likely to benefit from treatment, the definition of response, duration of treatment and cost-effectiveness.
Acknowledgments
The authors would like to thank Sydney ENT clinic staff and SydPath staff members, especially Tanya Wyatt and Peter Earls, for their support.
Footnote
Data Sharing Statement: Available at https://www.theajo.com/article/view/10.21037/ajo-23-28/dss
Peer Review File: Available at https://www.theajo.com/article/view/10.21037/ajo-23-28/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://www.theajo.com/article/view/10.21037/ajo-23-28/coif). R.J.H. serves as the Editor-in-Chief of Australian Journal of Otolaryngology. He is consultant and member of advisory board with Medtronic, Novartis and Meda pharmaceuticals. He has received research grant funding from Glaxo-Smith-Kline and Stallergenes. He also is on the speakers’ bureau for Glaxo-Smith-Kline, Astra-zeneca, Meda Pharmaceuticals and Seqirus. R.S. is consultant for Medtronic and expert witness for Avant Medical Defence. He is on the speaker’s bureau for Meda Pharmaceuticals. He is also President of Australian Society of Otolaryngology Head & Neck Surgery. L.H.K. is on the speakers’ bureau for Sequiris, Viatris, Stallergens, and Mylan and Care Pharmaceuticals. J.R. has been on the speakers’ bureau for Stallergenes, GSK, Astra Zeneca, and Sanofi. J.H. is supported by an Australian Government Research Training Program Scholarship, which is unrelated to the manuscript. R.G.C. serves as an unpaid editorial board member of Australian Journal of Otolaryngology from January 2019 to December 2024. She also has received payment for presentations/lectures from GSK. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study had ethics approval from the St Vincent’s Hospital Human Research Ethics Committee (REGIS ID 2019/PID04424) and participants provided written informed consent for data collection.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Li W, Ho J, Grayson JW, et al. Evaluation of Diffuse Type 2 Dominant or Eosinophilic Chronic Rhinosinusitis With Corticosteroid Irrigation After Surgical Neosinus Cavity Formation. JAMA Otolaryngol Head Neck Surg 2021;147:360-7. [Crossref] [PubMed]
- Fokkens WJ, Lund VJ, Hopkins C, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020;58:1-464. [Crossref] [PubMed]
- Ho J, Li W, Grayson JW, et al. Systemic medication requirement in post-surgical patients with eosinophilic chronic rhinosinusitis. Rhinology 2021;59:59-65. [PubMed]
- Walter S, Ho J, Alvarado R, et al. Effect of monoclonal antibody drug therapy on mucosal biomarkers in airway disease: A systematic review. Clin Exp Allergy 2020;50:1212-22. [Crossref] [PubMed]
- Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet 2012;380:651-9. [Crossref] [PubMed]
- Agache I, Akdis CA, Akdis M, et al. EAACI Biologicals Guidelines-Recommendations for severe asthma. Allergy 2021;76:14-44. [Crossref] [PubMed]
- Bachert C, Sousa AR, Lund VJ, et al. Reduced need for surgery in severe nasal polyposis with mepolizumab: Randomized trial. J Allergy Clin Immunol 2017;140:1024-1031.e14. [Crossref] [PubMed]
- Gevaert P, Van Bruaene N, Cattaert T, et al. Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. J Allergy Clin Immunol 2011;128:989-95.e1-8.
- Han JK, Bachert C, Fokkens W, et al. Mepolizumab for chronic rhinosinusitis with nasal polyps (SYNAPSE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med 2021;9:1141-53. [Crossref] [PubMed]
- Fokkens WJ, Lund V, Bachert C, et al. EUFOREA consensus on biologics for CRSwNP with or without asthma. Allergy 2019;74:2312-9. [Crossref] [PubMed]
- Ho J, Earls P, Harvey RJ. Systemic biomarkers of eosinophilic chronic rhinosinusitis. Curr Opin Allergy Clin Immunol 2020;20:23-9. [Crossref] [PubMed]
- Hopkins C, Gillett S, Slack R, et al. Psychometric validity of the 22-item Sinonasal Outcome Test. Clin Otolaryngol 2009;34:447-54. [Crossref] [PubMed]
- DeConde AS, Bodner TE, Mace JC, et al. Response shift in quality of life after endoscopic sinus surgery for chronic rhinosinusitis. JAMA Otolaryngol Head Neck Surg 2014;140:712-9. [Crossref] [PubMed]
- American Thoracic Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med 2005;171:912-30. [Crossref] [PubMed]
- Silkoff PE, Stevens A, Pak J, et al. A method for the standardized offline collection of exhaled nitric oxide. Chest 1999;116:754-9. [Crossref] [PubMed]
- Maniscalco M, Vitale C, Vatrella A, et al. Fractional exhaled nitric oxide-measuring devices: technology update. Med Devices (Auckl) 2016;9:151-60. [Crossref] [PubMed]
- Snidvongs K, Dalgorf D, Kalish L, et al. Modified Lund Mackay Postoperative Endoscopy Score for defining inflammatory burden in chronic rhinosinusitis. Rhinology 2014;52:53-9. [Crossref] [PubMed]
- Khatri S, Moore W, Gibson PG, et al. Assessment of the long-term safety of mepolizumab and durability of clinical response in patients with severe eosinophilic asthma. J Allergy Clin Immunol 2019;143:1742-1751.e7. [Crossref] [PubMed]
- Leung E, Al Efraij K, FitzGerald JM. The safety of mepolizumab for the treatment of asthma. Expert Opin Drug Saf 2017;16:397-404. [Crossref] [PubMed]
- Lugogo N, Domingo C, Chanez P, et al. Long-term Efficacy and Safety of Mepolizumab in Patients With Severe Eosinophilic Asthma: A Multi-center, Open-label, Phase IIIb Study. Clin Ther 2016;38:2058-2070.e1. [Crossref] [PubMed]
- Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med 2011;184:602-15. [Crossref] [PubMed]
- Rampersad A, Banerjee N, Hinks TSC. Are biologics for chronic rhinosinusitis effective and safe? Clin Exp Allergy 2021;51:870-2. [Crossref] [PubMed]
- Emma R, Morjaria JB, Fuochi V, et al. Mepolizumab in the management of severe eosinophilic asthma in adults: current evidence and practical experience. Ther Adv Respir Dis 2018;12:1753466618808490. [Crossref] [PubMed]
- Harvey ES, Langton D, Katelaris C, et al. Mepolizumab effectiveness and identification of super-responders in severe asthma. Eur Respir J 2020;55:1902420. [Crossref] [PubMed]
- Hopkins C, Rudmik L, Lund VJ. The predictive value of the preoperative Sinonasal Outcome Test-22 score in patients undergoing endoscopic sinus surgery for chronic rhinosinusitis. Laryngoscope 2015;125:1779-84. [Crossref] [PubMed]
- Wyrwich KW, Khan SA, Navaratnam P, et al. Validation and agreement across four versions of the asthma control questionnaire in patients with persistent asthma. Respir Med 2011;105:698-712. [Crossref] [PubMed]
- Juniper EF, Svensson K, Mörk AC, et al. Modification of the asthma quality of life questionnaire (standardised) for patients 12 years and older. Health Qual Life Outcomes 2005;3:58. [Crossref] [PubMed]
- Brescia G, Parrino D, Zanotti C, et al. Blood Eosinophil and Basophil Values Before and After Surgery for Eosinophilic-type Sinonasal Polyps. Am J Rhinol Allergy 2018;32:194-201. [Crossref] [PubMed]
- Drake VE, Rafaels N, Kim J. Peripheral blood eosinophilia correlates with hyperplastic nasal polyp growth. Int Forum Allergy Rhinol 2016;6:926-34. [Crossref] [PubMed]
- Honma A, Takagi D, Nakamaru Y, et al. Reduction of blood eosinophil counts in eosinophilic chronic rhinosinusitis after surgery. J Laryngol Otol 2016;130:1147-52. [Crossref] [PubMed]
- Walter S, Ho J, Alvarado R, et al. The effect of mepolizumab on local inflammatory biomarkers in eosinophilic chronic rhinosinusitis. Allergy 2021; In Press.
- Farah CS, Badal T, Reed N, et al. Mepolizumab improves small airway function in severe eosinophilic asthma. Respir Med 2019;148:49-53. [Crossref] [PubMed]
- Schleich F, Chaudhuri R, Canonica GW, et al. Mepolizumab improves lung function under real-world settings in REALITI-a study. Eur Respir J 2020;56:2260.
- Haldar P, Brightling CE, Singapuri A, et al. Outcomes after cessation of mepolizumab therapy in severe eosinophilic asthma: a 12-month follow-up analysis. J Allergy Clin Immunol 2014;133:921-3. [Crossref] [PubMed]
- Walford HH, Doherty TA. STAT6 and lung inflammation. JAKSTAT 2013;2:e25301. [Crossref] [PubMed]
- Brussino L, Heffler E, Bucca C, et al. Eosinophils Target Therapy for Severe Asthma: Critical Points. Biomed Res Int 2018;2018:7582057. [Crossref] [PubMed]
- Bjermer L, Alving K, Diamant Z, et al. Current evidence and future research needs for FeNO measurement in respiratory diseases. Respir Med 2014;108:830-41. [Crossref] [PubMed]
- Laugerud A, Jabeen M, Ramakrishnan S, et al. A proof-of-concept scale to predict asthma attacks: The OxfoRd Asthma attaCk risk ScaLE (ORACLE). American Thoracic Society International Conference 2021. p. A1436.
- Maniscalco M, Bianco A, Mazzarella G, et al. Recent Advances on Nitric Oxide in the Upper Airways. Curr Med Chem 2016;23:2736-45. [Crossref] [PubMed]
- Rimmer J, Hellings P, Lund VJ, et al. European position paper on diagnostic tools in rhinology. Rhinology 2019;57:1-41. [Crossref] [PubMed]
- Meltzer EO, Hamilos DL, Hadley JA, et al. Rhinosinusitis: developing guidance for clinical trials. J Allergy Clin Immunol 2006;118:S17-61. [Crossref] [PubMed]
- Ortega H, Lemiere C, Llanos JP, et al. Outcomes following mepolizumab treatment discontinuation: real-world experience from an open-label trial. Allergy Asthma Clin Immunol 2019;15:37. [Crossref] [PubMed]
Cite this article as: Ho J, Walter S, Alvarado R, Grayson JW, Campbell RG, Kalish LH, Sacks R, Sewell WA, Rimmer J, Harvey RJ. Clinical features of responders to mepolizumab in eosinophilic chronic rhinosinusitis and the outcomes post treatment cessation. Aust J Otolaryngol 2024;7:17.