
A diagnostic and surgical approach to masses of the buccal space: a case series
Introduction
The buccal space was first described by Juvara in 1870 (1), and subsequently by Coller and Yglesias in 1935 (2). Further detail regarding its contents and enclosing fasciae was elucidated by Gaughran in 1957 (3). Tumours of this space, although rare, represent a diverse range of pathologies. This is a consequence of the anatomic features of the space, including its close relationship with a variety of tissue types and communication with other spaces of the head and neck.
The surgical approach to a tumour within the buccal space depends largely on the location of the mass within the space and the index of suspicion of malignancy. As more accurate characterization of buccal space masses has become possible through the use of imaging and fine needle aspiration cytology (FNAC), surgical techniques have evolved to allow adequate surgical margins, while minimizing the risk of cosmetic defects. There are minimal surgical case series on the buccal space, the pathologies it includes and surgical approaches. Furthermore there is controversy regarding the optimal surgical approach to this space. Here, we present eleven cases of buccal space tumour that demonstrate our experience with a trans-oral surgical approach. We also present a review of the differential diagnoses, investigative approach and surgical management options for a buccal space mass.
Methods
A retrospective series of patients with buccal space masses was collected from a prospectively collated database of the two senior authors of this paper (F Riffat and C Palme). Eleven patients were identified with buccal space masses. Data was collected on basic demographic data (age, gender, comorbidities), investigations performed, surgical approach, pathological diagnosis and follow-up data. Cases were included if they arose primarily from the buccal space, whereas masses with invasion into the buccal space from surrounding spaces or oral mucosa [e.g., squamous cell carcinoma (SCC)] were excluded. Complications noted on follow-up were also recorded. Specifically, this included wound complications—bleeding and infection, and parotid duct or facial nerve injury.
Trans-oral surgical approach to buccal space masses
We access lesions of the buccal space via a trans-oral approach under general anaesthesia, using an oral nasotracheal tube, with the patient supine and neck extended. The use of a Boyle-Davis tonsil gag provides adequate access to the buccal space, and the approach can be further facilitated with the use of a cheek retractor. Lesions are excised with a Colorado monopolar dissection needle. This enables precise excision, with minimal tissue trauma, and haemostasis can be achieved at the same time. Suction tubing is taped to the cheek dissector to evacuate smoke.
A U-shaped mucosal incision is placed commencing anterior to Stensen’s orifice. The buccinator muscle has to be incised to access the buccal space. The facial artery supplying the nasolabial region is a direct branch of the external carotid artery that traverses the anterior compartment of the buccal space. The buccal artery, a branch of the maxillary artery supplying the posterior buccal space, enters posteromedially through the incomplete fascia and anastomoses with the facial artery. Both of these vessels are identified and clipped prior to excision of any pathological lesion.
The neural anatomy comprises the buccal branches of the facial and mandibular (V3) nerve. The former follows the parotid duct and has pierced the buccinators muscle in its final arborisations and hence is not injured by the dissection. The latter is responsible for internal mucosal sensation.
After appropriate haemostasis a capsular dissection of the lesion is performed taking care to avoid rupture with a blunt peanut dissection technique. The surgical assistant provides gentle external pressure to assist in tumour delivery. The incision is closed with interrupted 5.0 Vicryl Rapide sutures.
Patients are universally commenced on a soft diet within a day of surgery.
Results
Eleven patients with buccal space tumours were identified in our series (Table 1). The mean age was 40.6. There were 4 males and 7 females. All patients with lesions of the buccal space presented above had favourable short- and long-term post-operative outcomes, with no recurrence of original disease at final follow-up two years later. There were no post-operative complications and in particular there were no facial nerve or parotid duct injuries.
Table 1
| Case | Age | Sex | Pathology |
|---|---|---|---|
| 1 | 61 | F | Pleomorphic adenoma |
| 2 | 13 | F | Pleomorphic adenoma |
| 3 | 48 | M | Haemangioma |
| 4 | 74 | M | Masson’s haemangioma |
| 5 | 33 | F | Microcystic lymphatic malformation |
| 6 | 35 | F | Follicular lymphoma |
| 7 | 29 | F | Glomus tumour |
| 8 | 53 | F | Lipoma |
| 9 | 49 | M | Pleomorphic adenoma |
| 10 | 17 | F | Microcystic lymphatic malformation |
| 11 | 30 | F | Nodular fasciitis |
Case 1
A 61-year-old woman presented with a painless mass in the left cheek, noted during a routine dental examination. The mass was thought to be have been present for many years. A non-contrast magnetic resonance imaging (MRI) scan demonstrated a well-circumscribed lesion between the alveolar process of the left maxilla medially, the lateral wall of the left maxillary sinus and anterior surface of the ramus of the mandible posteriorly and the zygomatic process laterally; it showed low intensity signal on T1, and heterogeneous, high intensity signal on T2. FNAC was consistent with a pleomorphic adenoma, and post-operative histopathologic examination of the excised tissue confirmed this diagnosis.
Case 2
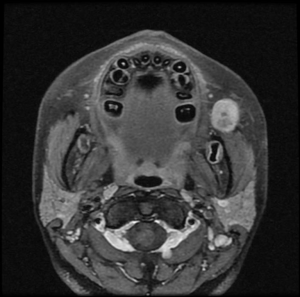
A 13-year-old female presented with a painless 21 mm diameter mass in the left buccal space. A well-circumscribed heterogeneous lesion was seen on a T1-weighted gadolinium-enhanced MRI scan (Figure 1). FNAC and histology confirmed a pleomorphic adenoma, with typical pseudopodia formation.
Case 3
A 48-year-old male presented with an asymptomatic, firm, mobile mass in the right cheek. MRI with gadolinium demonstrated a well-defined soft tissue mass anterior to the right buccinator, sandwiched between the buccinator, angle of the mandible and the lateral margin of the alveolus. It had a lower signal than parotid tissue and fat on T1, and a higher signal than that of parotid tissue on T2; fat suppression with gadolinium showed uniform, intense enhancement. FNAC yielded only blood. Histopathologic examination of the excised lesion showed a haemangioma characterized by well-demarcated lobules of dilated, thin-walled vascular spaces lined by endothelial cells, with bland cytologic features. There was some intervening fibrosis with chronic inflammation, and hemosiderin-laden macrophages consistent with previous haemorrhage. The post-operative course was uncomplicated.
Case 4
A 74-year-old male presented with an asymptomatic right cheek mass present for many months. On examination the mass was firm, non-tender and mobile within the right cheek.
Computed tomogram (CT) scan with contrast demonstrated a well-circumscribed, ovoid lesion overlying the right hemi-mandible, immediately anteroinferior to the right masseter. Intralesional calcification was present but there was no evidence of a focal destructive bony lesion, nor cervical lymphadenopathy. FNAC did not yield any diagnostic material. Post-operative histopathologic examination revealed a laminated organizing thrombus, partly surrounded by the wall of a dilated vessel, containing smooth muscle. The centre of the thrombus contained some calcification and reactive spindle-shaped fibroblasts in a fibrous stroma, with inflammation and signs of old haemorrhage. A diagnosis of Masson’s haemangioma (a papillary proliferation of thin-walled capillaries closely associated with thrombus) (4) was made.
Case 5
A 33-year-old female presented with a slow-growing painful 15 mm diameter mass in the left buccal space. A soft compressible mass was palpable on bimanual palpation. A T2-weighted MRI sequences demonstrated a multilocular cystic lesion with high signal intensity. Surgical excision confirmed a microcystic lymphatic malformation.
Case 6
A 35-year-old female presented with a painless, mobile left cheek mass palpable at the level of the parotid duct, present for several months. Significantly, she had a 4-year history of grade 1–2 follicular lymphoma affecting intra-abdominal nodes, for which she had received no active treatment. MRI with gadolinium revealed a trapezoidal lesion immediately anterior to the left masseter; on T1, the signal intensity was equal with that of muscle; there was minimal homogenous enhancement with gadolinium. Post-operative examination confirmed a diagnosis of grade 1–2 follicular lymphoma with predominantly a follicular and focal diffuse pattern. The neoplasm showed closely-packed follicles comprising small atypical CD20 positive B lymphocytes with rare scattered reactive CD3 positive T cells. The follicles demonstrated appropriate immunostaining with CD10 and BCL2. The Ki67 proliferation index was elevated.
Case 7
A 29-year-old female presented with a painful 23 mm diameter mass in the right buccal space. FNAC yielded sheets and cohesive clusters of relatively uniform appearing epithelioid cells with finely vacuolated cytoplasm and monotonous round nuclei with prominent pseudoinclusions. A diagnosis of a low-grade neoplasm, possibly of salivary gland origin was considered. Post-operative histopathologic examination showed an encapsulated, lobulated tumour comprising sheets of relatively uniform cells with interspersed delicate vascular channels. There was infiltration of the capsule, and mitotic activity was seen. An extensive panel of immunohistochemical stains performed on both the cell block as well as the resected lesion demonstrated immunoreactivity with smooth muscle actin (SMA), and were negative for a large number of epithelial, myoepithelial, vascular, melanocytic and lymphoid markers. A diagnosis of a glomus tumour of uncertain malignant potential was made in conjunction with international consultation (see Acknowledgments).
Case 8
A 53-year-old female presented with a non-tender discrete mobile mass in the left buccal space, present for several months. FNAC yielded lipocytes, consistent with lipoma. This diagnosis was borne out radiologically with a high intensity signal on T1-weighted MRI. The lesion was removed trans-orally, and the diagnosis of lipoma confirmed histologically.
Case 9
A 49-year-old male presented with a 7-year history of a palpable right buccal space mass that he was aware of for 7 years with a history of growth and pain over 6 months. FNAC demonstrated changes characteristic of an atypical pleomorphic adenoma. MRI T1 demonstrated gadolinium enhancement and lobulation. The lesion was removed successfully with a transoral extracapsular dissection confirming the histology.
Case 10
A 17-year-old female presented with an 8-month history of a soft, cystic mobile mass in the right buccal space. FNAC suggested lymphatic contents suggestive of a lymphatic malformation. T2-weighted MRI demonstrated a high homogenous signal consistent with the FNAC. The lesion was removed successfully with a transoral approach confirming the histology.
Case 11
A 30-year old female presented with a 2 cm asymptomatic mobile mass within the right cheek, subcutaneous and posterior to the oral commissure. It was hypointense on T1 and hyperintense on T2 and avidly enhancing. A FNAC suggested a pleomorphic adenoma. The patient underwent uncomplicated transoral excision. The final diagnosis was nodular fasciitis, a well circumscribed tumour with a thick capsule and bland spindle cells arranged in fascicles. There were no complications and no recurrence at last follow up.
Discussion
The buccal space is an incomplete anatomical fascial space bounded laterally by the superficial musculoaponeurotic system and muscles of fascial expression. Specifically, these are the zygomaticus major and minor and risorius. Anteromedially is the posterior surface of maxilla and buccinator and its attachment on the pterygomandibular raphe—an important conduit for spread of pathology between the buccal space and oropharynx (5). The posterior border is the edge of the mandibular ramus and muscles of mastication. Superiorly, the space is incomplete and communicates freely with the temporal fossa, while, inferiorly it is continuous with the submandibular space.
The bulk of the buccal space is occupied by the buccal fat pad, which is grooved by the parotid duct and divides the space into anterior and posterior compartments. The posterior compartment is identifiable as an area of lower attenuation on CT and higher fat signal on MRI compared to surrounding adipose tissue (6). In addition, the fat pad extends in four directions—laterally, along the parotid duct to the superficial lobe of the parotid gland; medially, between the mandible and maxillary sinus where it communicates with fat in the masticator space; superiorly, along the temporalis tendon; and anteriorly, superficial to the parotid duct. The main parotid duct (Stensen’s duct) traverses the buccal fat pad to pierce the buccinator muscle opposite the second molar, causing a retraction of the mucosa and submucosal fat (5).
Two major arteries lie within the buccal space. The facial artery supplying the nasolabial region is a direct branch of the external carotid artery that traverses the anterior compartment of the buccal space. The buccal artery, a branch of the maxillary artery supplying the posterior buccal space, enters posteromedially through the incomplete fascia and anastomoses with the facial artery. The facial vein is an important conduit for the spread of infection (5). It is located anterior to the parotid duct along the buccinator muscle and drains the nasolabial region to the deep facial vein and thence the cavernous sinus. Lymph nodes within the space drain the skin and subcutaneous tissues of the lower eyelid, nose and cheek and then drain to the submandibular nodes (7).
Two important nerves are also found within the buccal space—the buccal branch of the facial nerve originates within the parotid gland and runs parallel to the parotid duct, providing motor innervation to the buccinator and muscles of facial expression surrounding the buccal space (6); a branch of the mandibular nerve originates below the foramen ovale and enters the space posteromedially to supply sensation to the buccal mucosa. Finally, accessory parotid tissue and minor salivary glands are found within the buccal fat pad, lateral to the buccinators (8).
Masses within the buccal space are uncommon, and comprise a varied group of pathologies. Table 2 outlines the differential diagnosis of a buccal space mass, as well as the key radiologic and pathologic features of each lesion, as reported in the literature. Minor salivary gland neoplasms are the most common lesions reported (6). Haemangiomas and other vascular malformations are the next most commonly described masses (8). Abscesses are most likely to arise as a consequence of odontogenic infection, with buccal nodes and minor salivary glands less common sources of infection (11). Of note in this case series was the rarer diagnosis of a glomus tumour. Glomus tumours arise from glomus cells, which are modified smooth muscle cells located in the walls of specialized arteriovenous anastomoses (the Sucquet-Hoyer canal) involved in temperature regulation. These lesions are relatively common in the digital and subungual areas of young women and are known to be painful. Glomus tumours can occur rarely in mucosal and visceral locations. Clinically, the term ‘glomus’ may refer to a paraganglioma in the head and neck region; in histologic context it refers to a lesion arising from glomus cells.
Table 2
| Category | Diagnosis | Radiologic features | Pathologic features |
|---|---|---|---|
| Developmental lesions | Accessory parotid tissue | CT—anterior to the parotid hilum, overlying anterior margin of masseter; isodense with the parotid gland (9); more easily seen on CT than MRI (6) | Histologically identical to normal parotid gland |
| Dermoid cyst | CT—hypodense, thin-walled, well-circumscribed unilocular cyst (9). MRI—fat-fluid level or fat globule visible; high signal on T1 if fat content is high (9) | Cysts lined by stratified squamous epithelium with adnexal structures. The cysts may contain keratin or sebaceous material. Cyst rupture is associated with foreign body type giant cell response | |
| Infectious/inflammatory lesions | Abscess | CT—single or multi-loculated, hypodense lesion with peripheral rim enhancement; adjacent muscle enlargement and thickening of overlying skin; inflammatory changes in surrounding adipose tissue (9) | Acute inflammatory infiltrate with necrotic debris |
| Foreign body granuloma | CT—ill-defined infiltration of the buccal fat pad and subcutaneous fat pad; confined to subcutaneous tissue without extension into surrounding tissues; multiple punctate calcifications; fat density nodules; nodular calcification; often bilateral (10) | Multiple lipoid vacuolated spaces surrounded by multinucleated giant cells; background of dense fibrous tissue | |
| Kimura disease (more common in young Asian males) | MRI—variable high signal intensity on T2 depending on degree of fibrosis and vascular proliferation; strong enhancement on T1 (9) | Nodular, lymphoid follicular proliferation separated by fibrous septae. Dense infiltration of eosinophils | |
| Epidermoid cyst | CT—well-defined margin; adjacent to skin and distinct from buccinators (8) | Cysts lined by stratified squamous epithelium. Cyst contains keratin. Cyst rupture is associated with foreign body type giant cell response | |
| Vascular | Hemangioma | MRI—very high signal on T2, isointense with CSF; heterogeneous enhancement with contrast (6); flow voids seen on T1 and T2 images (9). CT—indistinguishable from other lesions unless phleboliths are seen (6) | Well delineated lobular lesion comprising ectatic vascular channels of varying shapes. The lining endothelial cells appear attenuated or show bland cytologic features |
| Arteriovenous malformation | MRI—characteristic serpiginous flow voids (9) | Several anastomotic thick and thin walled blood vessels. Interspersed fibroadipose tissue and nerve bundles may be present. Adjacent areas of scarring and hemosiderin laden macrophages due to previous episodes of bleeding | |
| Lymphangioma | MRI—cystic, septated lesion with fluid-fluid levels (9) | Ectatic lymphatic channels of varying shapes. The lining endothelial cells appear attenuated or show bland cytologic features | |
| Arterio-venous malformation | MRI—similar to hemangiomas; discrete areas of high signal intensity due to venous lakes, phleboliths (9) | Anastomosing thick and thin walled vascular structures of varying calibre. Areas of scarring and hemosiderin deposition following previous episodes of leakage and haemorrhage. Neural tissue may be present | |
| Lymphadenopathy | Reactive lymphadenopathy metastatic disease | CT—well- circumscribed mass with rim enhancement and low attenuation MRI—high signal on T2 (9) | Presence of metastatic epithelial/melanocytic cells depending upon the primary malignancy |
| Lymphoma | B-cell lymphoma—good demarcation, homogeneity, compression and moulding rather than invasion (3). T-cell lymphoma—non-specific imaging characteristics (9); MRI—signal intensity equal to muscle on T1 and greater than muscle on T2 (6) | Morphologic appearance variable as per the type and grade of lymphoma. Flow cytometry and immunophenotyping of the cells is essential for diagnosis | |
| Benign accessory parotid and minor salivary gland neoplasm | Pleomorphic adenoma | Well-circumscribed, rounded appearance. MRI—low signal on T1; high signal on T2 (9) | Well circumscribed lobulated lesion comprising epithelial and myoepithelial cells and mesenchymal matrix such as cartilaginous matrix |
| Monomorphic adenoma | CT—rim enhancement with central area of low attenuation (6) | Well circumscribed lobulated lesion comprising a single type of cells of salivary gland origin | |
| Malignant accessory parotid and minor salivary gland neoplasm | Adenoid cystic carcinoma | CT—homogeneous enhancement; isointense with muscle (6). MRI—higher signal intensity on T2 corresponds with lower cellularity and better prognosis (6) | Basaloid neoplasm comprising of cells arranged in nests with cribriform pattern. The cells show scanty to moderate amounts of cytoplasm and enlarged, hyperchromatic, angulated nuclei |
| Mucoepidermoid carcinoma | CT—heterogeneous enhancement with central area of low attenuation (6) | Variable histologic appearance depending upon the grade. The low grade tumors are predominantly cystic. The cystic spaces contain mucin and are lined by a mixture of goblet cells, cells with squamous appearance and intermediate cells. High grade tumors show more solid areas with fewer goblet cells | |
| Acinic cell carcinoma | – | Relatively well-delineated solid or cystic lesion comprising acinar cells with small nuclei and abundant intracytoplasmic zymogenic granules | |
| Carcinoma ex pleomorphic adenoma | CT—heterogeneously enhancing lesion. MRI—well-defined lesion with heterogeneous hypointensity on T1 and hyperintensity on T2 | Histologic appearance similar to pleomorphic adenoma with areas of infiltration into adjacent tissue and cytologic atypia, mitoses and necrosis | |
| Other benign neoplastic lesions | Lipoma | CT—isodense with subcutaneous fat (6). MRI—isointense with subcutaneous fat on all sequences (6) | Well-demarcated lesion comprising lobules of mature adipose tissue separated by fibrous tissue |
| Neurofibroma | MRI—high signal intensity on T2; homogeneously hyperintense or characteristic target sign of central hypointense region; strong contrast enhancement (9) | Unencapsulated, well demarcated lesion comprising of a mixture of spindle shaped neural cells and fibroblasts. Scanty mast cells are present. There is no cytologic atypia or mitoses | |
| Other benign neoplastic lesions | Schwannoma | – | Encapsulated lesion comprising spindle-shaped cells arranged in hyper and hypocellular areas (Antoni A and Antoni B) with organoid areas with peripheral palisading (Verocay bodies). The spindle shaped cells classically showy wavy goose neck nuclei and elongated cytoplasm. Several thick-walled hyalinised vessels are present |
| Rhabdomyosarcoma | CT—isodense with surrounding muscle (3). MRI—hyperintense on T2 compared to surrounding muscle; infiltration of surrounding tissues; variable degrees of heterogeneous contrast enhancement (9) | Histologic features vary with the type of rhabdomyosarcoma (embryonal, alveolar, botyroids, spindle, pleomorphic). The tumor may show variable proportions of sheets of undifferentiated small round blue cells and rhabdomyoblasts with abundant eosinophilic cytoplasm and eccentric pleomorphic nuclei. Spindle shaped strap cells with skeletal striations may also be present. Confirmatory immunohistochemistry with myogenin and desmin is essential. Fluorescent in situ hybridisation looking at specific translocations is of prognostic and predictive |
As illustrated by the cases presented above, the majority of buccal space lesions present as asymptomatic masses within the cheek, and clinical examination may not provide many clues as to their origin. Signs and symptoms suggestive of malignancy include a history of rapid growth, pain, ulceration of overlying skin or buccal mucosa, tethering of the mass to the skin or deeper structures and detection of lymphadenopathy in the head and neck region. Constitutional symptoms point to an infectious or inflammatory cause (6). The diagnostic approach to a buccal space mass involves careful clinical examination of the head and neck, imaging of the lesion and surrounding structures and, where feasible, an FNAC or biopsy of the mass.
The chief radiologic modalities employed in the evaluation of buccal space masses are CT and MRI, and the role of imaging is two-fold. Firstly, it is useful for determining the origin and relationships of the mass, and thus assists in surgical planning. In addition, knowledge of characteristic CT and MRI findings can be helpful in the diagnosis of specific lesions (Table 2). However, there are significant limitations to the diagnostic conclusions that can be drawn from imaging alone, particularly with regard to differentiating benign and malignant lesions. Many lesions also have non-specific soft tissue imaging characteristics (6).
It is well-recognized that malignant lesions within the buccal space can have smooth, well-circumscribed margins and, conversely, non-malignant lesions may be poorly defined (6,8,11). This is especially true of minor salivary gland neoplasms, and the sensitivity of MRI in predicting malignancy within the buccal space has been reported as between 29% (11) and 64% (8). Despite this, MRI is generally the preferred imaging modality as it provides superior soft tissue resolution, allowing better definition of the extent of invasive tumours. Features suggestive of malignancy include extension into adjoining spaces, with breach of fascial planes, bony destruction and associated lymphadenopathy (8). Where an inflammatory or infectious lesion is suspected, CT provides valuable information about inflammatory changes within the surrounding adipose tissue, and can be used to evaluate for CT-guided drainage of abscesses (6). The role of CT in differentiating benign and malignant lesions is more limited than that of MRI. The use of intravenous contrast, for both CT and MRI, is recommended to increase delineation of margins with respect to surrounding tissues, and to enable evaluation of vascularity of the mass (6).
In the present series, MRI was performed in all but one case where it was contraindicated. In each case, imaging characteristics were consistent with the ultimate diagnosis, but were not sufficient to obviate the need for more invasive diagnostic techniques. The primary role of imaging in these cases was in planning the surgical approach for resection. Ultrasound alone can be used to diagnose inflammatory conditions of the buccal space, including abscesses, often of an odontogenic etiology (12). Ultrasound has been shown to be effective in demonstrating spread of odontogenic infection into 76% of involved fascial spaces, with 100% concordance with MRI in identifying infection in 32 superficial spaces, including 13 buccal (13).
More recently, the role of Doppler ultrasound has been appraised as a tool for assessment of lesions within the buccal space. Ogura et al., in a study of 48 cases of buccal space lesions, showed that neoplastic and non-neoplastic lesions were significantly different with respect to heterogeneity and vascular signals, but not definition of margins or echogenicity (14). Overall, ultrasound was found to be useful in the evaluation of superficial structures within the buccal space, but due to lack of data on its sensitivity in the detection of malignant lesions, it cannot be recommended as the sole imaging modality where there is a high index of suspicion for malignancy. Given the advantages of ultrasound, including relative ease of availability and lack of radiation exposure, this is an important area for further investigation.
Examination by FNAC is an important step in the management of masses in the head and neck, its principal role being establishment of the need for surgery rather than definitive diagnosis. FNAC is the preferred initial step in the pathologic diagnosis of head and neck masses because of low rates of procedure-related morbidity and tumour seeding (15,16). While there is a paucity of data specifically assessing the accuracy of FNAC in the diagnosis of buccal space masses, a number of studies have examined its role in the management of parotid masses (15-17). In a major tertiary centre, the sensitivity and specificity of FNAC for diagnosis of malignancy in the parotid gland were 92% and 98%, respectively (15). However, the role of FNAC in soft tissue lesions, particularly vascular lesions of the buccal space is limited.
A number of surgical approaches to the buccal space have been described in the literature. Factors that influence the choice of approach are the need for adequate exposure, the ability to extend the surgical field intra-operatively if required, and cosmetic outcome (18). One of the earliest techniques described is the trans-cutaneous approach, in which a cutaneous incision is made directly over the lesion (19). Although this provides good exposure for a skin adnexal lesion, it does not allow adequate identification of the proximal facial nerve and parotid duct, thus deeper dissection risks injury to these structures. This approach is generally considered to be cosmetically unacceptable and has been largely abandoned.
The paranasal lip-split incision is another external approach to the buccal space that has been described. It allows wide exposure of the buccal space and cheek in a retrograde fashion (19) However, identification and isolation of the facial nerve is extremely difficult, limiting the utility of this approach. In addition, this incision results in a full-thickness cheek defect requiring flap reconstruction (20).
Where extensive exposure of the buccal space is required, for instance in the case of a suspected salivary gland malignancy, an extended parotidectomy approach is considered superior (18,21). This approach involves a pre-auricular incision that extends behind the ear and forward into the submandibular region (19). Superiorly it can be extended in the pre-auricular region to the hairline to increase exposure. Elevation of the skin flap allows complete exposure of the buccal space, enabling resection of the mass with adequate margins, as well as identification and isolation of the facial nerve branches and parotid duct. The incision can be further extended to allow more extensive lymph node dissection if required. Although this approach involves an external incision, good cosmesis is achieved. Disadvantages include risk of facial nerve neuropraxia from the need to dissection every division including terminal arborisations, seroma formation, salivary leak, loss of sensation to the earlobe as a result of greater auricular nerve damage and, rarely, Frey’s syndrome.
The trans-oral approach to masses within the buccal space that we have described is an approach which provides excellent cosmetic results at the expense of exposure. This limits its use to cases thought to be benign on imaging and biopsy. Kaneko and Kanai have proposed criteria for using the trans-oral approach: (I) preoperative diagnosis of a benign vascular lesion; (II) size <3 cm; (III) masses located anterior to the masseter (22). However, a number of other studies have reported success with this approach in the treatment of other pathologies, including pleomorphic adenomas (23,24), lipomas of the buccal fat pad (25,26), solitary fibrous tumors (27,28) and angiomyomas (29).
In cases deemed suitable for resection via a trans-oral approach, a direct approach to the tumour enables delivery into the operative field with gentle external pressure. Hence, it is our opinion that the lesion has to be freely mobile to bimanual palpation. The main disadvantage is the limited exposure that is achieved, restricting the size of the mass that can be resected and the margins achieved. Yamada et al. describe a technique utilizing a trans-oral approach combined with coronoidectomy to increase the surgical field, enabling resection of a larger mass located between the masseter and posterior wall of the maxillary sinus (30). Theoretical risks include post-operative trismus and malocclusion.
Conclusions
Tumours of the buccal space are a rare entity. This paper describes the clinicopathologic characteristics of these neoplasms and the importance of cytology and radiology in formulating a diagnosis. The role of the trans-oral approach in complete resection of these neoplasms, with minimal long-term morbidity and risk of recurrence, is highlighted.
Acknowledgments
We wish to thank Professor Bruce Wenig, Chairman, Department of Pathology and Laboratory Medicine, Beth Israel Medical Center and St. Luke’s and Roosevelt Hospitals, New York for his help with the diagnosis of glomus tumour of uncertain malignant potential. Sincere thanks to Dr. Dvung Vu for cadaveric dissection assistance.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ajo.2019.05.05). FR serves as an unpaid editorial board member of Australian Journal of Otolaryngology. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Juvara E. Anatomie de la région ptérygo-maxillaire. Paris: Bataille, 1895:65.
- Coller FA, Yglesias L. Infections of the lip and face. Surg Gynecol Obstet 1935;60:277-90.
- Gaughran GR. Fasciae of the Masticator Space. Anat Rec 1957;129:383-400. [Crossref] [PubMed]
- Narwal A, Sen R, Singh V, et al. Masson’s haemangioma: a rare intraoral presentation. Contemp Clin Dent 2013;4:397-401. [Crossref] [PubMed]
- Seelagan D, Noujaim SE. A pictorial review of the anatomy and common pathology of the buccal space: “the overlooked space Applied Radiol 2007;36:20-8.
- Tart RP, Kotzur IM, Mancuso AA, et al. CT and MR imaging of the buccal space and buccal space masses. Radiographics 1995;15:531-50. [Crossref] [PubMed]
- Tart RP, Mukherji SK, Avino AJ, et al. Facial lymph nodes: normal and abnormal CT appearance. Radiology 1993;188:695-700. [Crossref] [PubMed]
- Kurabayashi T, Ida M, Yoshin N, et al. Computed tomography of buccal space masses. Dentomaxillofac Radiol 1997;26:347-53. [Crossref] [PubMed]
- Kim HC, Han MH, Moon MH, et al. CT and MR imaging of the buccal space: normal anatomy and abnormalities. Korean J Radiol 2005;6:22-30. [Crossref] [PubMed]
- Gu DH, Yoon DY, Chang SK, et al. CT features of foreign body granulomas after cosmetic paraffin injection into the cervicofacial area. Diagn Interv Radiol 2010;16:125-8. [PubMed]
- Kurabayashi T, Ida M, Tetsumura A, et al. MR imaging of benign and malignant lesions in the buccal space. Dentomaxillofac Radiol 2002;31:344-9. [Crossref] [PubMed]
- Srinivas K, Sumanth KN, Chopra SS. Ultrasonographic evaluation of inflammatory swellings of buccal space. Indian J Dent Res 2009;20:458-62. [Crossref] [PubMed]
- Bassiony M, Yang J, Abdel-Monem TM, et al. Exploration of ultrasonography in assessment of fascial space spread of odontogenic infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009;107:861-9. [Crossref] [PubMed]
- Ogura I, Kaneda T, Sasaki Y, et al. Characteristic power Doppler sonographic images of tumorous and non-tumorous buccal space lesions. Dentomaxillofac Radiol 2013;42:20120460. [Crossref] [PubMed]
- Carrillo JF, Ramirez R, Flores L, et al. Diagnostic accuracy of fine needle aspiration biopsy in preoperative diagnosis of patients with parotid gland masses. J Surg Oncol 2009;100:133-8. [Crossref] [PubMed]
- Seethala RR, Livolsi VA, Baloch ZW. Relative accuracy of fine needle aspiration and frozen section in the diagnosis of lesions of the parotid gland. Head Neck 2005;27:217-23. [Crossref] [PubMed]
- Das DK, Petkar MA, Al Mane NM, et al. Role of fine needle aspiration cytology in the diagnosis of swellings in the salivary gland regions: a study of 712 cases. Med Princ Pract 2004;13:95-106. [Crossref] [PubMed]
- Rodgers GK, Myers EN. Surgical management of the mass in the buccal space. Laryngoscope 1988;98:749-53. [Crossref] [PubMed]
- Gallia L, Rood SR, Myers EN. Management of buccal space masses. Otolaryngol Head Neck Surg 1981;89:221-5. [Crossref] [PubMed]
- Landa LE, Kathju S, Nepomuceno-Perez MC, et al. Tuberculous granuloma and adenoid cystic carcinoma presenting as a single buccal space mass. J Craniofac Surg 2002;13:533-7. [Crossref] [PubMed]
- Sano K, Fujita S, Sekine J, et al. Metachronous manifestation of carcinoma ex pleomorphic adenoma in a buccal minor salivary gland and the contralateral parotid gland: a case report and review of the literature. J Oral Maxillofac Surg 2012;70:2701-12. [Crossref] [PubMed]
- Kaneko K, Kanai R. Cavernous haemangioma of the accessory parotid gland. J Craniofac Surg 2011;22:e28-9. [Crossref] [PubMed]
- Lou SMY, Rich AM, De Silva RK, et al. Pleomorphic adenoma of a molar salivary gland. Oral Oncol Extra 2006;42:170-2. [Crossref]
- Nakamori K, Ohuchi T, Hasegawa T, et al. Carcinoma ex pleomorphic adenoma of the buccal region is composed of salivary duct carcinoma and squamous cell carcinoma components. Int J Oral Maxillofac Surg 2009;38:1116-8. [Crossref] [PubMed]
- de Wijn RS, van der Heijden EP, Kon M. On lipoma of the buccal fat pad: report of two cases and review of the literature. J Plast Reconstr Aesthet Surg 2009;62:28-35. [Crossref] [PubMed]
- Brucoli M, Arcuri F, Borello G, et al. Surgical technique of the transoral approach to remove a lipoma of the buccal fat pad. J Craniofac Surg 2011;22:2415-8. [Crossref] [PubMed]
- Shin JH, Sung IY, Suh JH, et al. Solitary fibrous tumour in the buccal space: MR findings with pathologic correlation. AJNR Am J Neuroradiol 2001;22:1890-2. [PubMed]
- Fusconi M, Ciofalo A, Greco A, et al. Solitary fibrous tumour of the oral cavity: case report and pathologic consideration. J Oral Maxillofac Surg 2008;66:530-4. [Crossref] [PubMed]
- Kim HY, Jung SN, Kwon H, et al. Angiomyoma in the buccal space. J Craniofac Surg 2010;21:1634-5. [Crossref] [PubMed]
- Yamada H, Hamada Y, Fujihara H, et al. Solitary fibrous tumour of the buccal space resected in combination with coronoidectomy. Oral Surg Oral Med Oral Pathol Oral Radiol 2012;114:e9-14. [Crossref] [PubMed]
Cite this article as: Riffat F, Hasan Z, Buchanan M, Vu D, Palme C. A diagnostic and surgical approach to masses of the buccal space: a case series. Aust J Otolaryngol 2019;2:18.


